| << Chapter < Page | Chapter >> Page > |
Several classes of inorganic and metalorganic sources have been explored as copper sources. Inorganic precursors for copper CVD used hydrogen reduction of copper halide sources of the type CuX or CuX 2 , where X is chlorine (Cl) or fluorine (F):
2 CuX + H 2 → 2 Cu + 2 HX
CuX 2 + H 2 → Cu + 2 HX
The volatility of copper halides is low, the reactions involved require prohibitively high temperatures (400 - 1200 °C), lead to the production of corrosive by-products such as hydrochloric and hydrofluoric acids (HCl and HF), and produce deposits with large concentrations of halide contaminants. Meanwhile, the exploration of metalorganic chemistries has involved various copper(II) and copper(I) source precursors, with significant advantages over inorganic precursors.
Copper was known to form very few stable, volatile alkyl or carbonyl compounds. This was thought to eliminate the two major classes of compounds used in most existing processes for CVD of metals or compound semiconductors. Copper halides have been used for chemical vapor transport growth of Cu-containing semiconductor crystals. But the evaporation temperatures needed for copper halides are much higher than those needed for metal-organic compounds. Film purity and resistivity were also a problem, possibly reflecting the high reactivity of Si substrates with metal halides.
Cu(II) compounds that have been studied as CVD precursors are listed in [link] . The structural formulas of these compounds are shown in [link] along with the ligand abbreviations in [link] . Each compound contains a central Cu(II) atom bonded to two singly charged β-diketonate or β-ketoiminate ligands. Most of them are stable, easy to synthesize, transport and handle.
| Compound | Evaporation temp. (°C) | Deposition temp. (°C) | Carrier gas | Reactor pressure (Torr) |
| Cu(acac) 2 | 180 - 200 | 225 - 250 | H 2 /Ar | 760 |
| Cu(hfac) 2 | 80 - 95 | 250 - 300 | H 2 | 760 |
| Cu(tfac) 2 | 135 - 160 | 250 - 300 | H 2 | 760 |
| Cu(dpm) 2 | 100 | 400 | none | <10 -2 |
| Cu(ppm) 2 | 100 | 400 | none | <0.3 |
| Cu(fod) 2 | - | 300 - 400 | H 2 | 10 -3 - 760 |
| Cu(acim) 2 | 287 | 400 | H 2 | 730 |
| Cu(nona-F) 2 | 85 - 105 | 270 - 350 | H 2 | 10 - 70 |
| Cu(acen) 2 | 204 | 450 | H 2 | 730 |
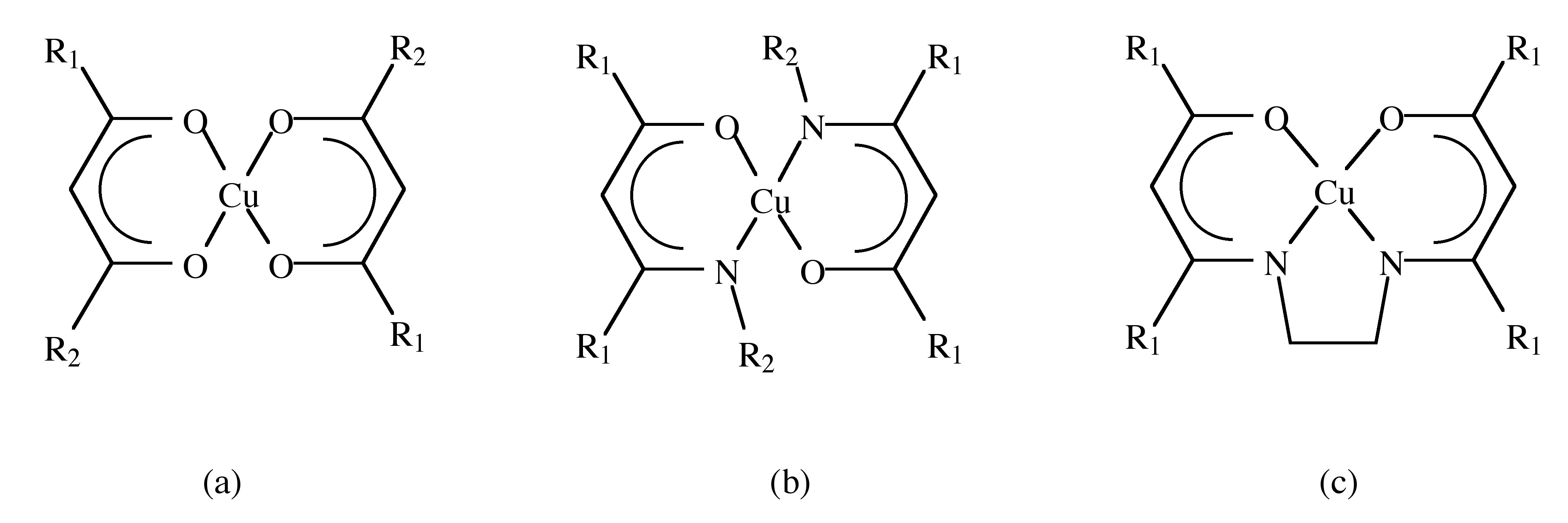
| Ligand abbreviation | R 1 | R 2 | Structural type |
| acac | CH 3 | CH 3 | a |
| hfac | CF 3 | CF 3 | a |
| tfac | CH 3 | CF 3 | a |
| dpm | C(CH 3 ) 3 | C(CH 3 ) 3 | a |
| ppm | C(CH 3 ) 3 | CF 2 CF 3 | a |
| fod | C(CH 3 ) 3 | CF 2 CF 2 CF 3 | a |
| acim | CH 3 | H | b |
| nona-F | CF 3 | CH 2 CF 3 | b |
| acen | CH 3 | - | c |
Attention has focused on Cu(II) β-diketonate [i.e., Cu(tfac) 2 , Cu(hfac) 2 ] and Cu(II) β-ketoiminate [i.e., Cu(acim) 2 , Cu(acen) 2 ]. An important characteristic of Cu(II) compounds as CVD precursors is the use of heavily fluorinated ligand such as Cu(tfac) 2 and Cu(hfac) 2 versus Cu(acac) 2 . The main effort of fluorine substitution is a significant increase in the volatility of the complex.
Cu(hfac) 2 is by far the most extensively studied of the Cu(II) CVD precursors. Preparations in aqueous solutions yield the yellow-green dihydrate, Cu(hfac) 2 ·2H 2 O. This is stable in very humid air or at lower temperatures but slowly loses one molecule of water under typical laboratory conditions to form the “grass-green” monohydrate, Cu(hfac) 2 ·H 2 O. The monohydrate, which is commercially available, can be sublimed unchanged and melts at 133 – 136 °C. More vigorous drying over concentrated H 2 SO 4 produces the purple anhydrous compound Cu(hfac) 2 (mp = 95 – 98 °C). The purple material is hydroscopic, converting readily into the monohydrate. Other β-diketonate Cu(II) complexes are prepared by the similar method.

Notification Switch
Would you like to follow the 'Chemistry of electronic materials' conversation and receive update notifications?