| << Chapter < Page | Chapter >> Page > |
Most people are familiar with carbohydrates, one type of macromolecule, especially when it comes to what we eat. To lose weight, some individuals adhere to “low-carb” diets. Athletes, in contrast, often “carb-load” before important competitions to ensure that they have enough energy to compete at a high level. Carbohydrates are, in fact, an essential part of our diet; grains, fruits, and vegetables are all natural sources of carbohydrates. Carbohydrates provide energy to the body, particularly through glucose, a simple sugar that is a component of starch and an ingredient in many staple foods. Carbohydrates also have other important functions in humans, animals, and plants.
Carbohydrates can be represented by the stoichiometric formula (CH 2 O) n , where n is the number of carbons in the molecule. In other words, the ratio of carbon to hydrogen to oxygen is 1:2:1 in carbohydrate molecules. This formula also explains the origin of the term “carbohydrate”: the components are carbon (“carbo”) and the components of water (hence, “hydrate”). Carbohydrates are classified into three subtypes: monosaccharides, disaccharides, and polysaccharides.
Monosaccharides (mono- = “one”; sacchar- = “sweet”) are simple sugars, the most common of which is glucose. In monosaccharides, the number of carbons usually ranges from three to seven. Most monosaccharide names end with the suffix -ose. If the sugar has an aldehyde group (the functional group with the structure R-CHO), it is known as an aldose, and if it has a ketone group (the functional group with the structure RC(=O)R'), it is known as a ketose. Depending on the number of carbons in the sugar, they also may be known as trioses (three carbons), pentoses (five carbons), and or hexoses (six carbons). See [link] for an illustration of the monosaccharides.
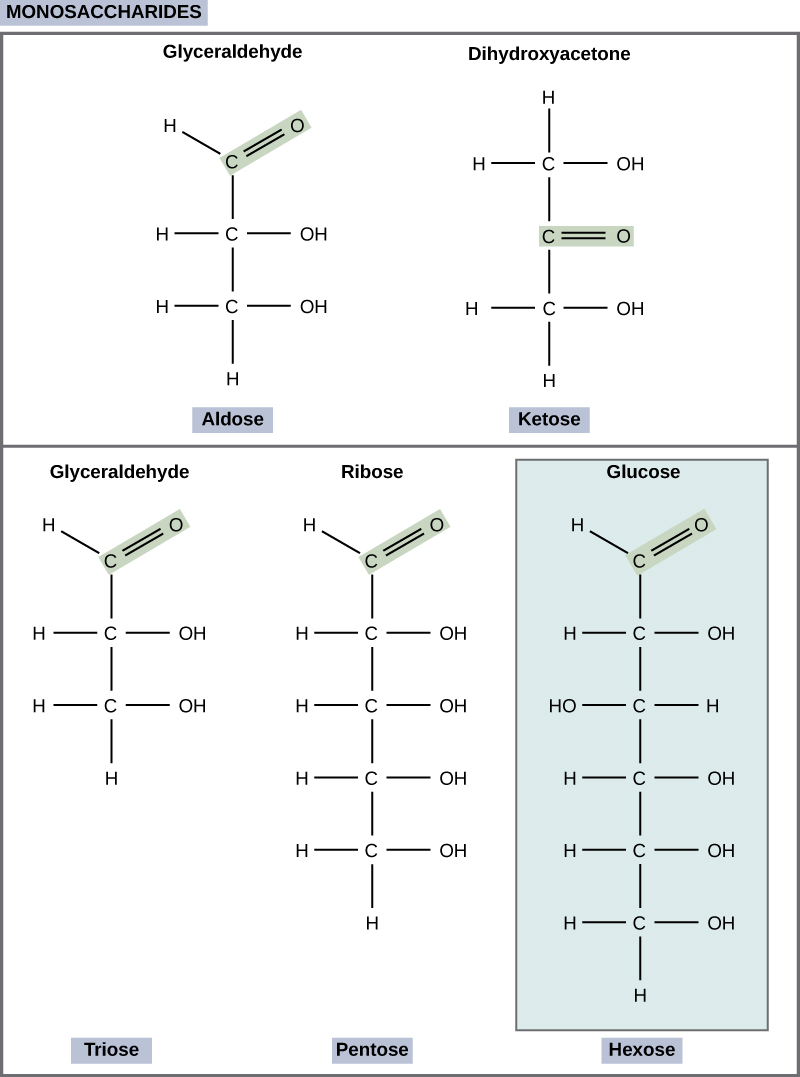
The chemical formula for glucose is C 6 H 12 O 6 . In humans, glucose is an important source of energy. During cellular respiration, energy is released from glucose, and that energy is used to help make adenosine triphosphate (ATP). Plants synthesize glucose using carbon dioxide and water, and glucose in turn is used for energy requirements for the plant. Excess glucose is often stored as starch that is catabolized (the breakdown of larger molecules by cells) by humans and other animals that feed on plants.
Galactose (part of lactose, or milk sugar) and fructose (found in sucrose, in fruit) are other common monosaccharides. Although glucose, galactose, and fructose all have the same chemical formula (C 6 H 12 O 6 ), they differ structurally and chemically (and are known as isomers) because of the different arrangement of functional groups around the asymmetric carbon; all of these monosaccharides have more than one asymmetric carbon ( [link] ).

Notification Switch
Would you like to follow the 'Biology' conversation and receive update notifications?