| << Chapter < Page | Chapter >> Page > |
| Aquo ion | n |
| [Li(H 2 O) n ] + | 4 |
| [Na(H 2 O) n ] + | 6 |
| [K(H 2 O) n ] + | 6 |
| [Rb(H 2 O) n ] + | 6 - 8 |
| [Cs(H 2 O) n ] + | 8 |
In general the alkali metal ions form complexes with hard donor such as oxygen (H 2 O, ROH, RCO 2 - , etc.) or nitrogen (e.g., NH 3 , NR 3 , etc.). The aquo complexes readily exchange the water for other ligands, [link] ; however, the equilibrium constants are small when the ligand is similar in size to water.
As a consequence of the low equilibrium constants for monodentate ligands, the alkali metal cations, M + , favor coordination to polydentate ligands such as ethylenediaminetetraacetic acid (EDTA, [link] ), polyethers, and even natural polyesters or polypeptides. In each case the polydentate ligand wraps itself around the cation.
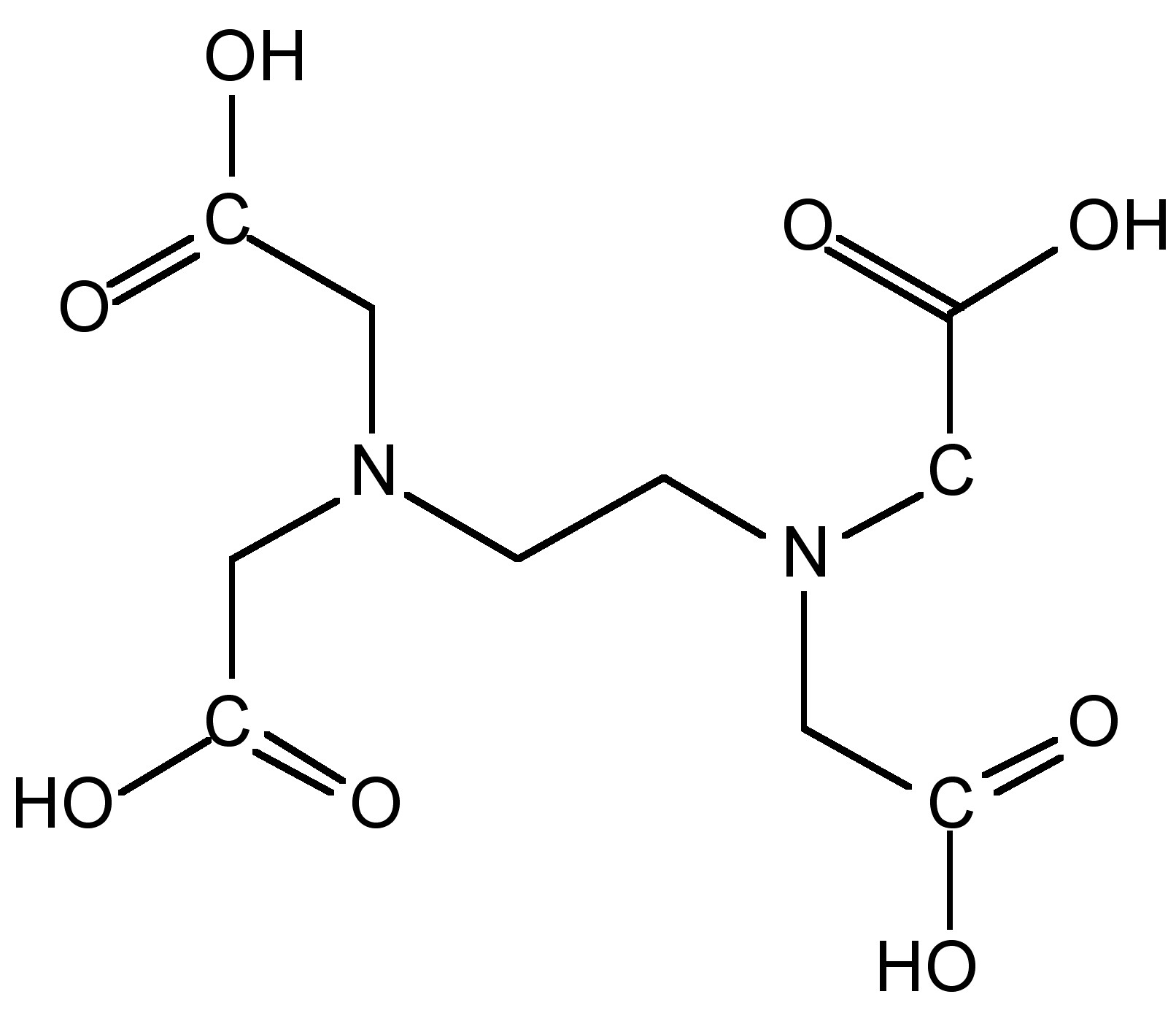
Macrocyclic ligands represent a special class of polydentate ligand. They are defined as being a cyclic compound with nine or more members including all the heteroatoms and with three or more donor atoms. and are given the special name of cryptands when they are synthetic bi- and poly-cyclic multidentate ligands. Crown ethers is the name applied to macrocyclic ligands that consist of a ring containing several ether groups. [link] shows several common macrocyclic ligands.
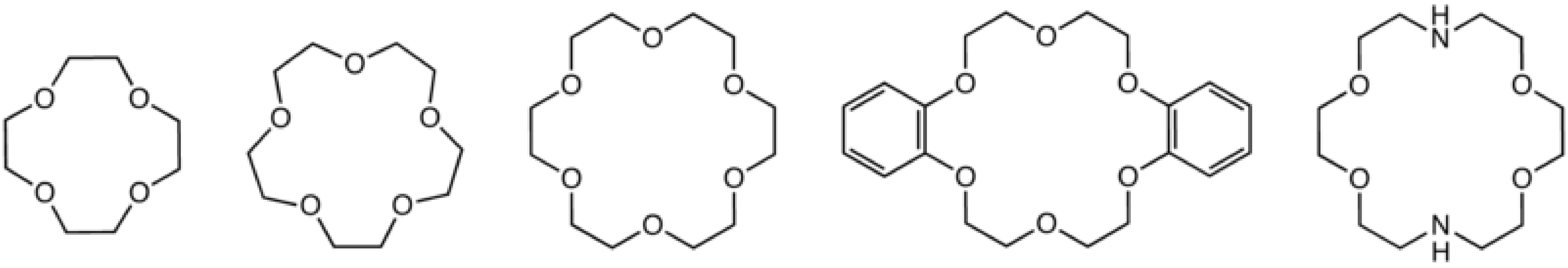
Macrocyclic ligands are generally oxygen or nitrogen donor ligands, and they form highly stable 1:1 complexes with alkali metal ions in which all or most of the coordination sites on the metal are occupied, i.e., [M(L)] + rather than [M(L)(H 2 O) n ] + . Since the external surface of the macrocyclic ligands comprises of organic residue (e.g., CH 2 groups) the complexes are soluble in organic solvents. Thus, crown ethers are commonly used to solubilize salts (e.g., NaCl) in organic solvents. They have also been used to create solutions of nanoparticles, such as carbon nanotubes ( [link] ), that are ordinarily highly insoluble in common organic solvents ( [link] ).
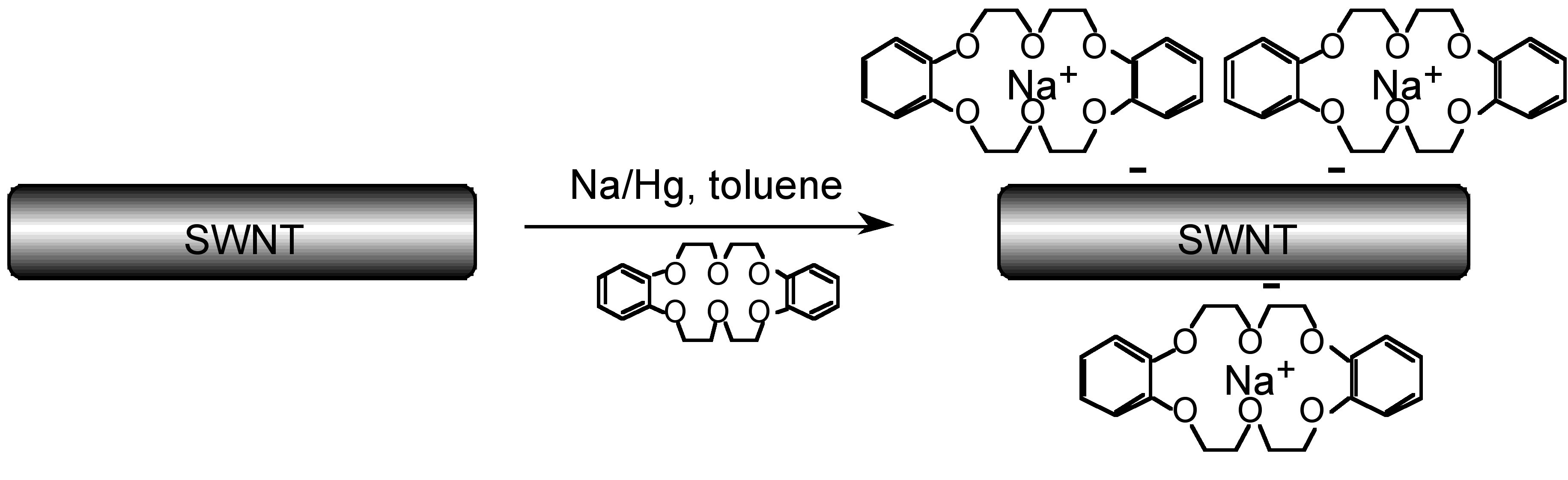
| Solvent | SWNT concentration (mg/L) |
| CH 2 Cl 2 | 14.05 |
| DMF | 11.42 |
| hexane | 4.65 |
| toluene | 3.79 |
| EtOH | 2.37 |
| MeOH | 2.37 |
| CHCl 3 | 0.30 |
| H 2 O | 0.10 |
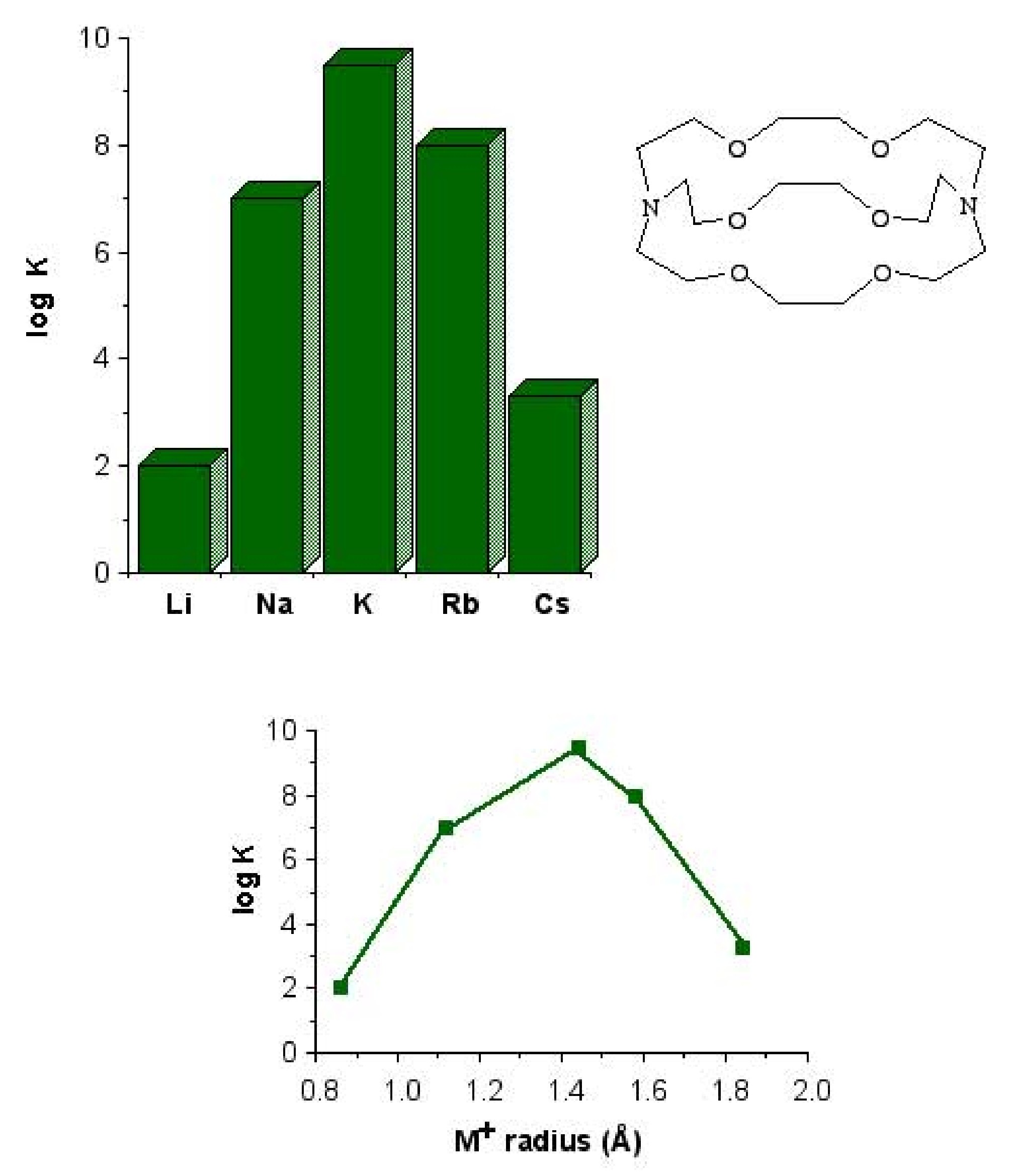
The most important factor in the coordination of various macrocyclic ligands to alkali metal ions is the relative size of the ion and the ligand cavity. For example, 4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8,8,8]hexacosane is a potentially octa-dentate ligand; however, the binding efficiency is very dependant on the identity of the M + ion ( [link] ). The low binding constant for lithium is probably as a consequence that the ligand would have to distort to coordinate to the small cation. Conversely, the lower equilibrium for caesium is due to its being to large to fit completely into the ligand cavity.
One application of the size effect for macrocyclic ligands is the ability to selectively bind different metals. For example, the 4,7,13,16,21-pentaoxa-1,10-diazabicyclo[8,8,5]tricosane and 4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8,8,8]hexacosane ligands shown in [link] have very different binding constants to Na + and K + , as a consequence of the relative size of the cation and the ligand cavity ( [link] ).
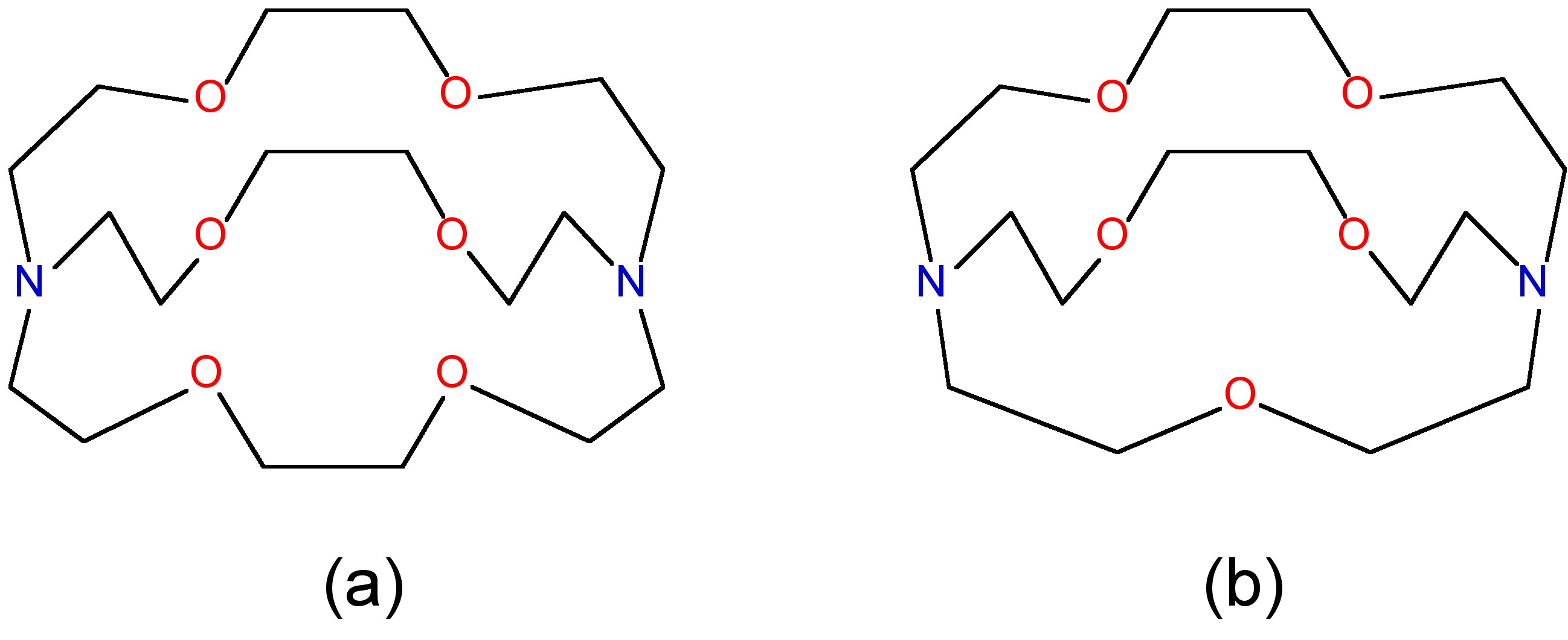
| Cation | [2,2,2] | [2,2,1] |
| Na + | 800 | 250,000 |
| K + | 250,000 | 700 |

Notification Switch
Would you like to follow the 'Chemistry of the main group elements' conversation and receive update notifications?