| << Chapter < Page | Chapter >> Page > |
Calcium carbonate reacts with sulfurous acid as follows:
In a polluted atmosphere where the concentration of sulfur dioxide is high, calcium carbonate deteriorates more rapidly than in less polluted air. Similarly, phosphorus burns much more rapidly in an atmosphere of pure oxygen than in air, which is only about 20% oxygen.

Phosphorous burns rapidly in air, but it will burn even more rapidly if the concentration of oxygen in is higher. Watch this video to see an example.
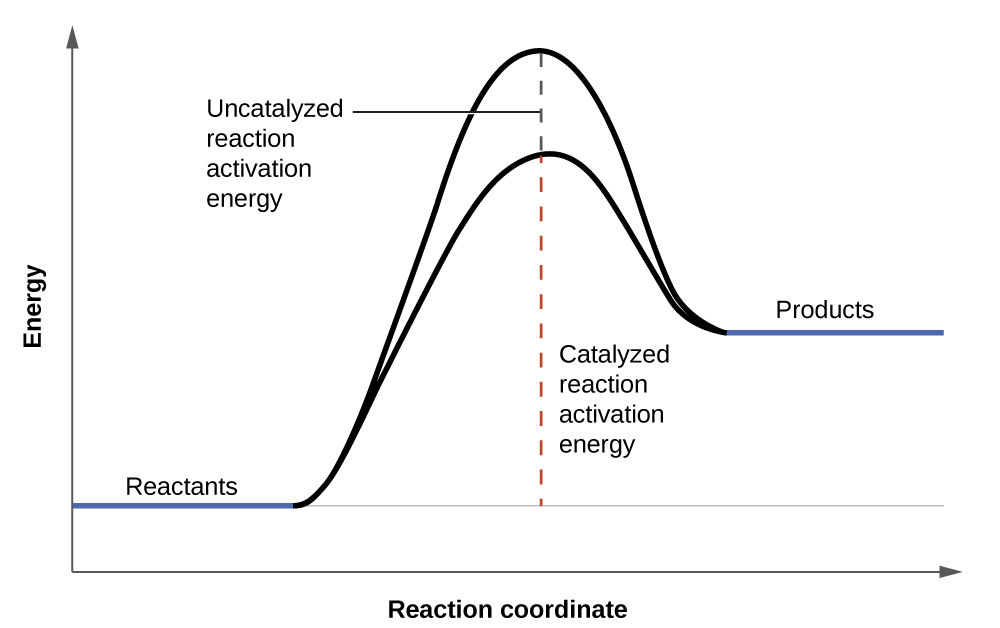
Hydrogen peroxide solutions foam when poured onto an open wound because substances in the exposed tissues act as catalysts, increasing the rate of hydrogen peroxide’s decomposition. However, in the absence of these catalysts (for example, in the bottle in the medicine cabinet) complete decomposition can take months. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without itself being consumed by the reaction. Activation energy is the minimum amount of energy required for a chemical reaction to proceed in the forward direction. A catalyst increases the reaction rate by providing an alternative pathway or mechanism for the reaction to follow ( [link] ). Catalysis will be discussed in greater detail later in this chapter as it relates to mechanisms of reactions.
Chemical reactions occur when molecules collide with each other and undergo a chemical transformation. Before physically performing a reaction in a laboratory, scientists can use molecular modeling simulations to predict how the parameters discussed earlier will influence the rate of a reaction. Use the PhET Reactions&Rates interactive to explore how temperature, concentration, and the nature of the reactants affect reaction rates.
The rate of a chemical reaction is affected by several parameters. Reactions involving two phases proceed more rapidly when there is greater surface area contact. If temperature or reactant concentration is increased, the rate of a given reaction generally increases as well. A catalyst can increase the rate of a reaction by providing an alternative pathway that causes the activation energy of the reaction to decrease.
Describe the effect of each of the following on the rate of the reaction of magnesium metal with a solution of hydrochloric acid: the molarity of the hydrochloric acid, the temperature of the solution, and the size of the pieces of magnesium.
Higher molarity increases the rate of the reaction. Higher temperature increases the rate of the reaction. Smaller pieces of magnesium metal will react more rapidly than larger pieces because more reactive surface exists.
Explain why an egg cooks more slowly in boiling water in Denver than in New York City. (Hint: Consider the effect of temperature on reaction rate and the effect of pressure on boiling point.)
Go to the PhET Reactions&Rates interactive. Use the Single Collision tab to represent how the collision between monatomic oxygen (O) and carbon monoxide (CO) results in the breaking of one bond and the formation of another. Pull back on the red plunger to release the atom and observe the results. Then, click on “Reload Launcher” and change to “Angled shot” to see the difference.
(a) What happens when the angle of the collision is changed?
(b) Explain how this is relevant to rate of reaction.
(a) Depending on the angle selected, the atom may take a long time to collide with the molecule and, when a collision does occur, it may not result in the breaking of the bond and the forming of the other. (b) Particles of reactant must come into contact with each other before they can react.
In the PhET Reactions&Rates interactive, use the “Many Collisions” tab to observe how multiple atoms and molecules interact under varying conditions. Select a molecule to pump into the chamber. Set the initial temperature and select the current amounts of each reactant. Select “Show bonds” under Options. How is the rate of the reaction affected by concentration and temperature?
In the PhET Reactions&Rates interactive, on the Many Collisions tab, set up a simulation with 15 molecules of A and 10 molecules of BC. Select “Show Bonds” under Options.
(a) Leave the Initial Temperature at the default setting. Observe the reaction. Is the rate of reaction fast or slow?
(b) Click “Pause” and then “Reset All,” and then enter 15 molecules of A and 10 molecules of BC once again. Select “Show Bonds” under Options. This time, increase the initial temperature until, on the graph, the total average energy line is completely above the potential energy curve. Describe what happens to the reaction.
(a) very slow; (b) As the temperature is increased, the reaction proceeds at a faster rate. The amount of reactants decreases, and the amount of products increases. After a while, there is a roughly equal amount of BC , AB , and C in the mixture and a slight excess of A .

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?