| << Chapter < Page | Chapter >> Page > |
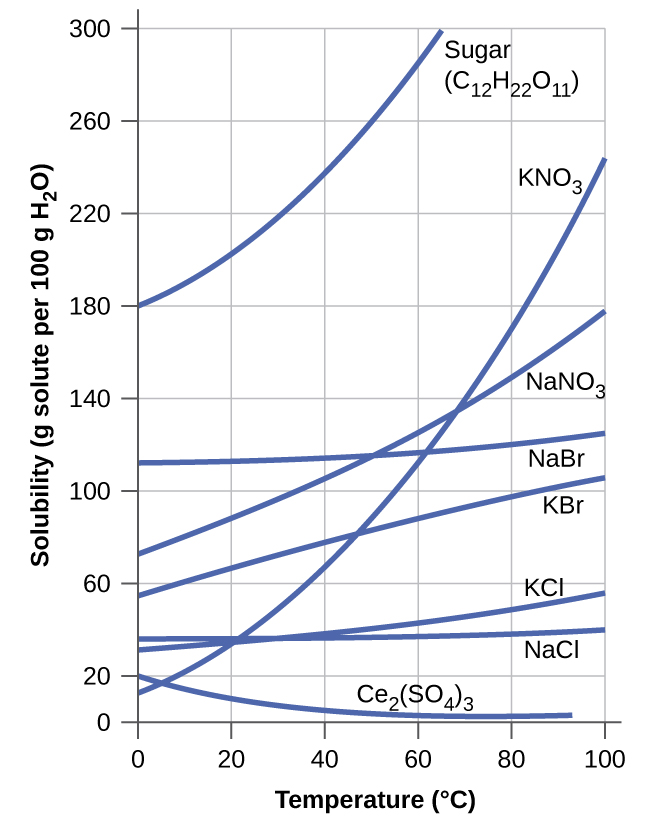
The dependence of solubility on temperature for a number of inorganic solids in water is shown by the solubility curves in [link] . Reviewing these data indicate a general trend of increasing solubility with temperature, although there are exceptions, as illustrated by the ionic compound cerium sulfate.
The temperature dependence of solubility can be exploited to prepare supersaturated solutions of certain compounds. A solution may be saturated with the compound at an elevated temperature (where the solute is more soluble) and subsequently cooled to a lower temperature without precipitating the solute. The resultant solution contains solute at a concentration greater than its equilibrium solubility at the lower temperature (i.e., it is supersaturated) and is relatively stable. Precipitation of the excess solute can be initiated by adding a seed crystal (see the video in the Link to Learning earlier in this module) or by mechanically agitating the solution. Some hand warmers, such as the one pictured in [link] , take advantage of this behavior.

This video shows the crystallization process occurring in a hand warmer.
The extent to which one substance will dissolve in another is determined by several factors, including the types and relative strengths of intermolecular attractive forces that may exist between the substances’ atoms, ions, or molecules. This tendency to dissolve is quantified as substance’s solubility, its maximum concentration in a solution at equilibrium under specified conditions. A saturated solution contains solute at a concentration equal to its solubility. A supersaturated solution is one in which a solute’s concentration exceeds its solubility—a nonequilibrium (unstable) condition that will result in solute precipitation when the solution is appropriately perturbed. Miscible liquids are soluble in all proportions, and immiscible liquids exhibit very low mutual solubility. Solubilities for gaseous solutes decrease with increasing temperature, while those for most, but not all, solid solutes increase with temperature. The concentration of a gaseous solute in a solution is proportional to the partial pressure of the gas to which the solution is exposed, a relation known as Henry’s law.
Suppose you are presented with a clear solution of sodium thiosulfate, Na 2 S 2 O 3 . How could you determine whether the solution is unsaturated, saturated, or supersaturated?
Supersaturated solutions of most solids in water are prepared by cooling saturated solutions. Supersaturated solutions of most gases in water are prepared by heating saturated solutions. Explain the reasons for the difference in the two procedures.
The solubility of solids usually decreases upon cooling a solution, while the solubility of gases usually decreases upon heating.
Suggest an explanation for the observations that ethanol, C 2 H 5 OH, is completely miscible with water and that ethanethiol, C 2 H 5 SH, is soluble only to the extent of 1.5 g per 100 mL of water.
Calculate the percent by mass of KBr in a saturated solution of KBr in water at 10 °C. See [link] for useful data, and report the computed percentage to one significant digit.
40%
Which of the following gases is expected to be most soluble in water? Explain your reasoning.
(a) CH 4
(b) CCl 4
(c) CHCl 3
At 0 °C and 1.00 atm, as much as 0.70 g of O 2 can dissolve in 1 L of water. At 0 °C and 4.00 atm, how many grams of O 2 dissolve in 1 L of water?
2.80 g
Refer to [link] .
(a) How did the concentration of dissolved CO 2 in the beverage change when the bottle was opened?
(b) What caused this change?
(c) Is the beverage unsaturated, saturated, or supersaturated with CO 2 ?
The Henry’s law constant for CO 2 is 3.4 10 −2 M /atm at 25 °C. What pressure of carbon dioxide is needed to maintain a CO 2 concentration of 0.10 M in a can of lemon-lime soda?
2.9 atm
The Henry’s law constant for O 2 is 1.3 10 −3 M /atm at 25 °C. What mass of oxygen would be dissolved in a 40-L aquarium at 25 °C, assuming an atmospheric pressure of 1.00 atm, and that the partial pressure of O 2 is 0.21 atm?
How many liters of HCl gas, measured at 30.0 °C and 745 torr, are required to prepare 1.25 L of a 3.20- M solution of hydrochloric acid?
102 L HCl

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?