| << Chapter < Page | Chapter >> Page > |
Now consider fluorine (F), a component of bones and teeth. Its atomic number is nine, and it has seven electrons in its valence shell. Thus, it is highly likely to bond with other atoms in such a way that fluorine accepts one electron (it is easier for fluorine to gain one electron than to donate seven electrons). When it does, its electrons will outnumber its protons by one, and it will have an overall negative charge. The ionized form of fluorine is called fluoride, and is written as F – . A negatively charged ion is known as an anion .
Atoms that have more than one electron to donate or accept will end up with stronger positive or negative charges. A cation that has donated two electrons has a net charge of +2. Using magnesium (Mg) as an example, this can be written Mg ++ or Mg 2+ . An anion that has accepted two electrons has a net charge of –2. The ionic form of selenium (Se), for example, is typically written Se 2– .
The opposite charges of cations and anions exert a moderately strong mutual attraction that keeps the atoms in close proximity forming an ionic bond. An ionic bond is an ongoing, close association between ions of opposite charge. The table salt you sprinkle on your food owes its existence to ionic bonding. As shown in [link] , sodium commonly donates an electron to chlorine, becoming the cation Na + . When chlorine accepts the electron, it becomes the chloride anion, Cl – . With their opposing charges, these two ions strongly attract each other.
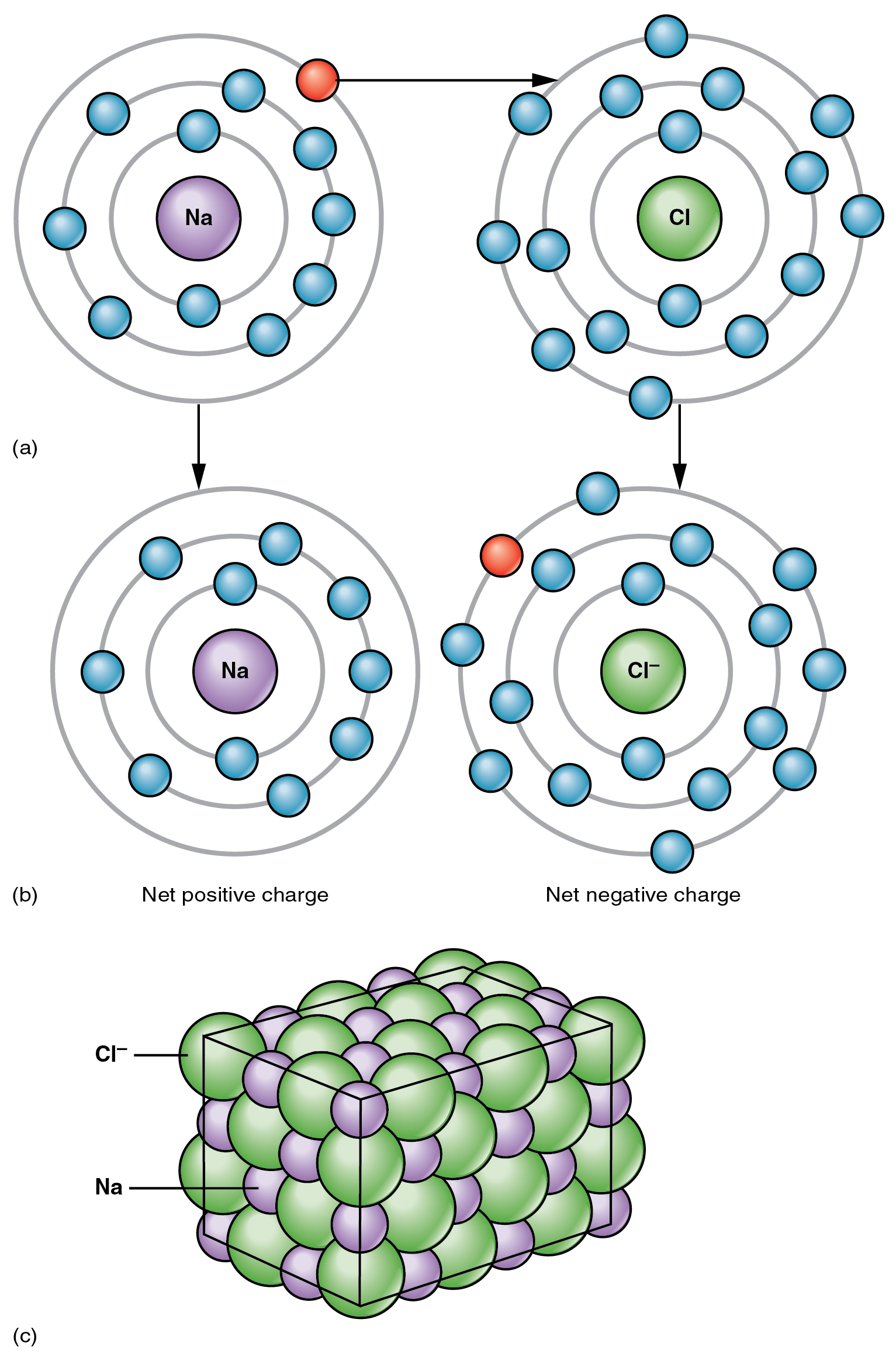
Water is an essential component of life because it is able to break the ionic bonds in salts to free the ions. In fact, in biological fluids, most individual atoms exist as ions. These dissolved ions produce electrical charges within the body. The behavior of these ions produces the tracings of heart and brain function observed as waves on an electrocardiogram (EKG or ECG) or an electroencephalogram (EEG). The electrical activity that derives from the interactions of the charged ions is why they are also called electrolytes.
Unlike ionic bonds formed by the attraction between a cation’s positive charge and an anion’s negative charge, molecules formed by a covalent bond share electrons in a mutually stabilizing relationship. Like next-door neighbors whose kids hang out first at one home and then at the other, the atoms do not lose or gain electrons permanently. Instead, the electrons move back and forth between the elements. Because of the close sharing of pairs of electrons (one electron from each of two atoms), covalent bonds are stronger than ionic bonds.
[link] shows several common types of covalent bonds. Notice that the two covalently bonded atoms typically share just one or two electron pairs, though larger sharings are possible. The important concept to take from this is that in covalent bonds, electrons in the outermost valence shell are shared to fill the valence shells of both atoms, ultimately stabilizing both of the atoms involved. In a single covalent bond, a single electron is shared between two atoms, while in a double covalent bond, two pairs of electrons are shared between two atoms. There even are triple covalent bonds, where three atoms are shared.

Notification Switch
Would you like to follow the 'Anatomy & Physiology' conversation and receive update notifications?