| << Chapter < Page | Chapter >> Page > |
Lithium (Li), whose atomic number is 3, has three electrons. Two of these fill the first electron shell, and the third spills over into a second shell. The second electron shell can accommodate as many as eight electrons. Carbon, with its six electrons, entirely fills its first shell, and half-fills its second. With ten electrons, neon (Ne) entirely fills its two electron shells. Again, a look at the periodic table reveals that all of the elements in the second row, from lithium to neon, have just two electron shells. Atoms with more than ten electrons require more than two shells. These elements occupy the third and subsequent rows of the periodic table.
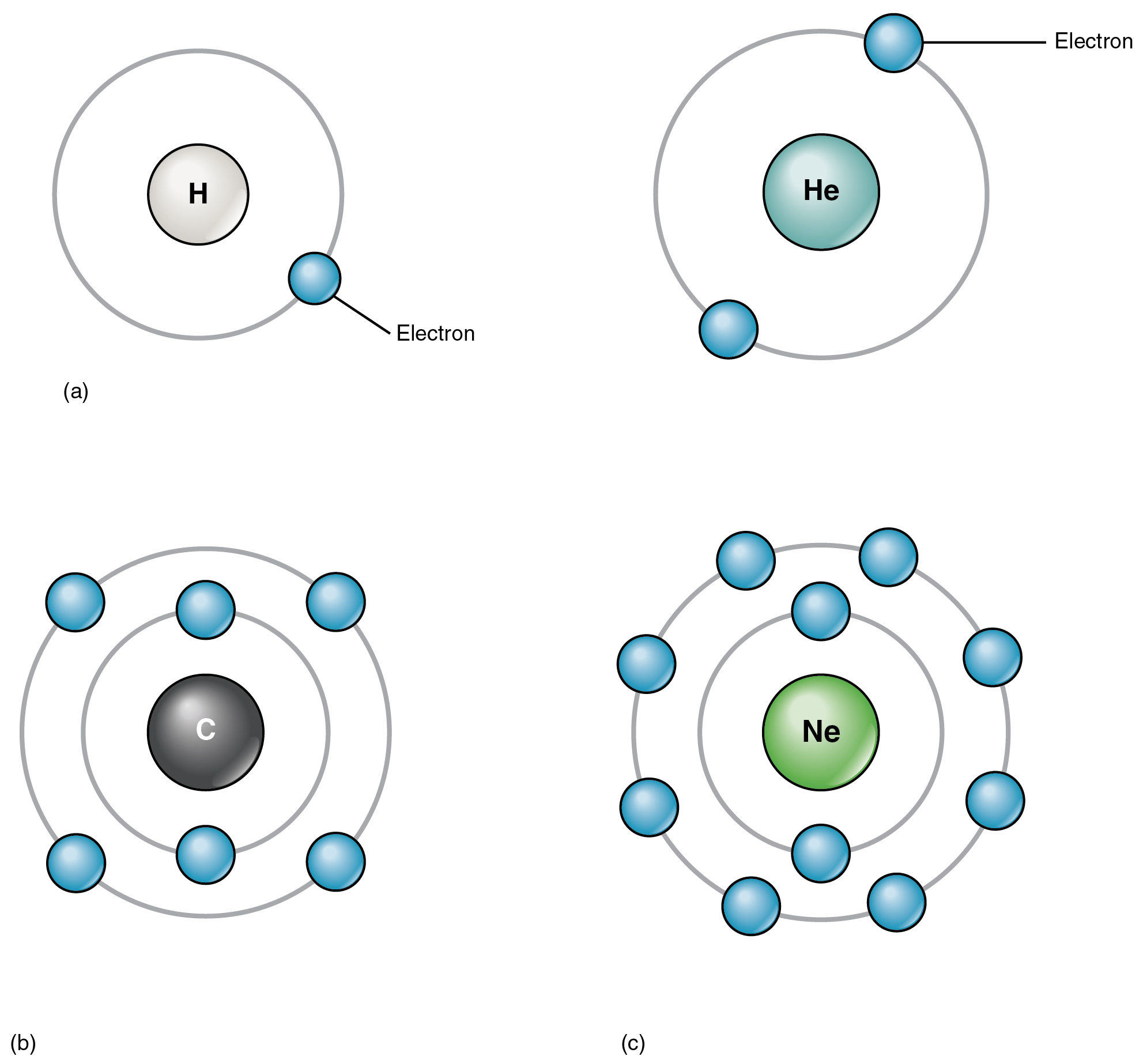
The factor that most strongly governs the tendency of an atom to participate in chemical reactions is the number of electrons in its valence shell. A valence shell is an atom’s outermost electron shell. If the valence shell is full, the atom is stable; meaning its electrons are unlikely to be pulled away from the nucleus by the electrical charge of other atoms. If the valence shell is not full, the atom is reactive; meaning it will tend to react with other atoms in ways that make the valence shell full. Consider hydrogen, with its one electron only half-filling its valence shell. This single electron is likely to be drawn into relationships with the atoms of other elements, so that hydrogen’s single valence shell can be stabilized.
All atoms (except hydrogen and helium with their single electron shells) are most stable when there are exactly eight electrons in their valence shell. This principle is referred to as the octet rule, and it states that an atom will give up, gain, or share electrons with another atom so that it ends up with eight electrons in its own valence shell. For example, oxygen, with six electrons in its valence shell, is likely to react with other atoms in a way that results in the addition of two electrons to oxygen’s valence shell, bringing the number to eight. When two hydrogen atoms each share their single electron with oxygen, covalent bonds are formed, resulting in a molecule of water, H 2 O.
In nature, atoms of one element tend to join with atoms of other elements in characteristic ways. For example, carbon commonly fills its valence shell by linking up with four atoms of hydrogen. In so doing, the two elements form the simplest of organic molecules, methane, which also is one of the most abundant and stable carbon-containing compounds on Earth. As stated above, another example is water; oxygen needs two electrons to fill its valence shell. It commonly interacts with two atoms of hydrogen, forming H 2 O. Incidentally, the name “hydrogen” reflects its contribution to water (hydro- = “water”; -gen = “maker”). Thus, hydrogen is the “water maker.”
The human body is composed of elements, the most abundant of which are oxygen (O), carbon (C), hydrogen (H) and nitrogen (N). You obtain these elements from the foods you eat and the air you breathe. The smallest unit of an element that retains all of the properties of that element is an atom. But, atoms themselves contain many subatomic particles, the three most important of which are protons, neutrons, and electrons. These particles do not vary in quality from one element to another; rather, what gives an element its distinctive identification is the quantity of its protons, called its atomic number. Protons and neutrons contribute nearly all of an atom’s mass; the number of protons and neutrons is an element’s mass number. Heavier and lighter versions of the same element can occur in nature because these versions have different numbers of neutrons. Different versions of an element are called isotopes.
The tendency of an atom to be stable or to react readily with other atoms is largely due to the behavior of the electrons within the atom’s outermost electron shell, called its valence shell. Helium, as well as larger atoms with eight electrons in their valence shell, is unlikely to participate in chemical reactions because they are stable. All other atoms tend to accept, donate, or share electrons in a process that brings the electrons in their valence shell to eight (or in the case of hydrogen, to two).
Visit this website to view the periodic table. In the periodic table of the elements, elements in a single column have the same number of electrons that can participate in a chemical reaction. These electrons are known as “valence electrons.” For example, the elements in the first column all have a single valence electron—an electron that can be “donated” in a chemical reaction with another atom. What is the meaning of a mass number shown in parentheses?
The mass number is the total number of protons and neutrons in the nucleus of an atom.

Notification Switch
Would you like to follow the 'Anatomy & Physiology' conversation and receive update notifications?