| << Chapter < Page | Chapter >> Page > |
Ion selective electrode (ISE) is an analytical technique used to determine the activity of ions in aqueous solution by measuring the electrical potential. ISE has many advantages compared to other techniques, including:
Based on these advantages, ISE has wide variety of applications, which is reasonable considering the importance of measuring ion activity. For example, ISE finds its use in pollution monitoring in natural waters (CN - , F - , S - , Cl - , etc.), food processing (NO 3 - , NO 2 - in meat preservatives), Ca 2+ in dairy products, and K + in fruit juices, etc.
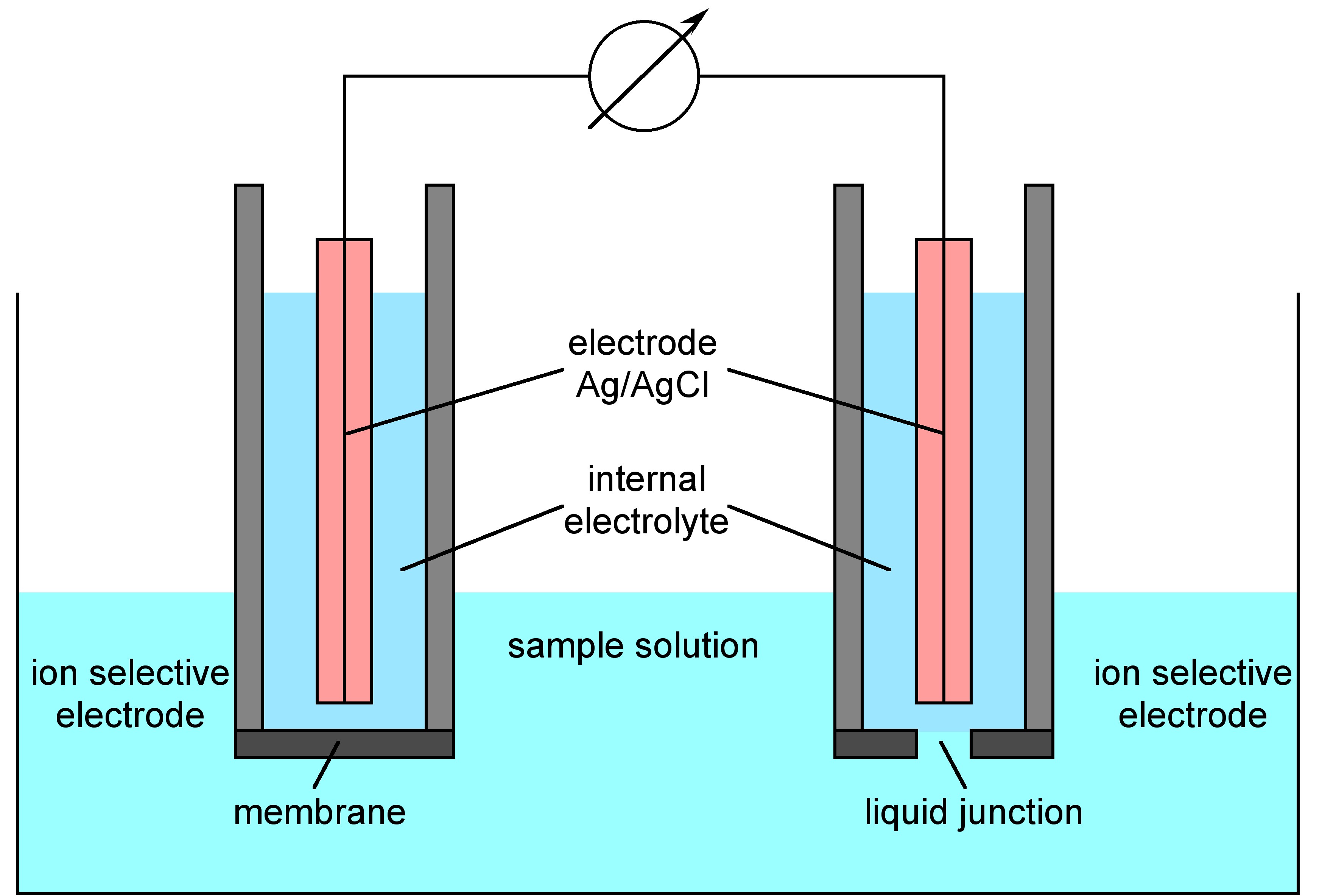
Before focusing on how ISE works, it would be good to get an idea what ISE setup looks like and the component of the ISE instrument. [link] shows the basic components of ISE setup. It has an ion selective electrode, which allows measured ions to pass, but excludes the passage of the other ions. Within this ion selective electrode, there is an internal reference electrode ( [link] ), which is made of silver wire coated with solid silver chloride, embedded in concentrated potassium chloride solution (filling solution) saturated with silver chloride. This solution also contains the same ions as that to be measured. There is also a reference electrode similar to ion selective electrode, but there is no to-be-measured ion in the internal electrolyte and the selective membrane is replaced by porous frit, which allows the slow passage of the internal filling solution and forms the liquid junction with the external text solution. The ion selective electrode and reference electrode are connected by a milli-voltmeter. Measurment is accomplished simply by immersing the two electrodes in the same test solution.
There are commonly more than one types of ions in solution. So how ISE manage to measure the concentration of certain ion in solution without being affected by other ions? This is done by applying a selective membrane at the ion selective electrode, which only allows the desired ion to go in and out. At equilibrium, there is potential difference existing between two sides of the membrane, and it is governed by the concentration of the tested solution described by Nernst equation EQ, where E is potential, E 0 is a constant characteristic of a particular ISE, R is the gas constant (8.314 J/K.mol), T is the temperature (in K), n is the charge of the ion and F is Faraday constant (96,500 coulombs/mol). To make it relevant, the measured potential difference is proportional to the logarithm of ion concentration. Thus, the relationship between potential difference and ion concentration can be determined by measuring the potential of two solutions of already-known ion concentration and a plot based on the measured potential and logarithm of the ion concentration. Based on this plot, the ion concentration of an unknown solution can be known by measuring the potential and corresponding it to the plot.

Notification Switch
Would you like to follow the 'Physical methods in chemistry and nano science' conversation and receive update notifications?