| << Chapter < Page | Chapter >> Page > |
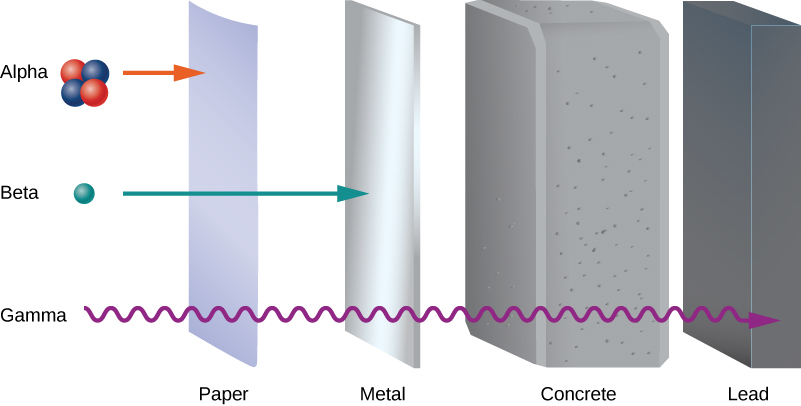
Early experiments revealed three types of nuclear “rays” or radiation: alpha ( ) rays , beta ( ) rays , and gamma ( ) rays . These three types of radiation are differentiated by their ability to penetrate matter. Alpha radiation is barely able to pass through a thin sheet of paper. Beta radiation can penetrate aluminum to a depth of about 3 mm, and gamma radiation can penetrate lead to a depth of 2 or more centimeters ( [link] ).
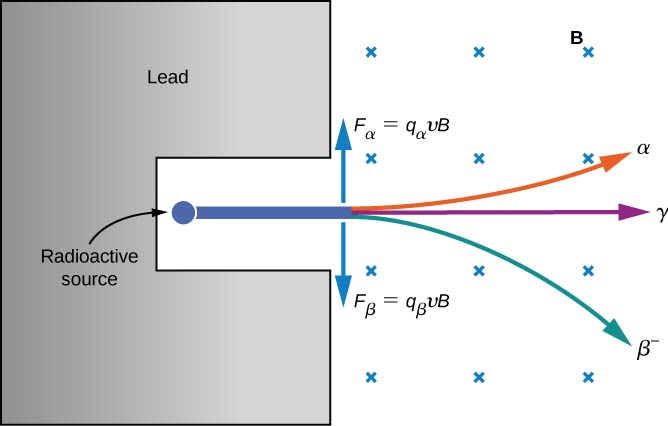
The electrical properties of these three types of radiation are investigated by passing them through a uniform magnetic field, as shown in [link] . According to the magnetic force equation positively charged particles are deflected upward, negatively charged particles are deflected downward, and particles with no charge pass through the magnetic field undeflected. Eventually, rays were identified with helium nuclei rays with electrons and positrons (positively charged electrons or antielectrons ), and rays with high-energy photons. We discuss alpha, beta, and gamma radiation in detail in the remainder of this section.
Heavy unstable nuclei emit radiation. In -particle decay (or alpha decay ), the nucleus loses two protons and two neutrons, so the atomic number decreases by two, whereas its mass number decreases by four. Before the decay, the nucleus is called the parent nucleus . The nucleus or nuclei produced in the decay are referred to as the daughter nucleus or daughter nuclei. We represent an decay symbolically by
where is the parent nucleus, is the daughter nucleus, and is the particle. In decay, a nucleus of atomic number Z decays into a nucleus of atomic number and atomic mass Interestingly, the dream of the ancient alchemists to turn other metals into gold is scientifically feasible through the alpha-decay process. The efforts of the alchemists failed because they relied on chemical interactions rather than nuclear interactions.
Watch alpha particles escape from a polonium nucleus, causing radioactive alpha decay. See how random decay times relate to the half-life. To try a simulation of alpha decay, visit alpha particles
An example of alpha decay is uranium-238:
The atomic number has dropped from 92 to 90. The chemical element with is thorium. Hence, Uranium-238 has decayed to Thorium-234 by the emission of an particle, written
Subsequently, decays by emission with a half-life of 24 days. The energy released in this alpha decay takes the form of kinetic energies of the thorium and helium nuclei, although the kinetic energy of thorium is smaller than helium due to its heavier mass and smaller velocity.

Notification Switch
Would you like to follow the 'University physics volume 3' conversation and receive update notifications?