| << Chapter < Page | Chapter >> Page > |
The recognition and appreciation of patterns has enabled us to make many discoveries. The periodic table of elements was proposed as an organized summary of the known elements long before all elements had been discovered, and it led to many other discoveries. We shall see in later chapters that patterns in the properties of subatomic particles led to the proposal of quarks as their underlying structure, an idea that is still bearing fruit.
Knowledge of the properties of elements and compounds grew, culminating in the mid-19th-century development of the periodic table of the elements by Dmitri Mendeleev (1834–1907), the great Russian chemist. Mendeleev proposed an ingenious array that highlighted the periodic nature of the properties of elements. Believing in the systematics of the periodic table, he also predicted the existence of then-unknown elements to complete it. Once these elements were discovered and determined to have properties predicted by Mendeleev, his periodic table became universally accepted.
Also during the 19th century, the kinetic theory of gases was developed. Kinetic theory is based on the existence of atoms and molecules in random thermal motion and provides a microscopic explanation of the gas laws, heat transfer, and thermodynamics (see Introduction to Temperature, Kinetic Theory, and the Gas Laws and Introduction to Laws of Thermodynamics ). Kinetic theory works so well that it is another strong indication of the existence of atoms. But it is still indirect evidence—individual atoms and molecules had not been observed. There were heated debates about the validity of kinetic theory until direct evidence of atoms was obtained.
The first truly direct evidence of atoms is credited to Robert Brown, a Scottish botanist. In 1827, he noticed that tiny pollen grains suspended in still water moved about in complex paths. This can be observed with a microscope for any small particles in a fluid. The motion is caused by the random thermal motions of fluid molecules colliding with particles in the fluid, and it is now called Brownian motion . (See [link] .) Statistical fluctuations in the numbers of molecules striking the sides of a visible particle cause it to move first this way, then that. Although the molecules cannot be directly observed, their effects on the particle can be. By examining Brownian motion, the size of molecules can be calculated. The smaller and more numerous they are, the smaller the fluctuations in the numbers striking different sides.
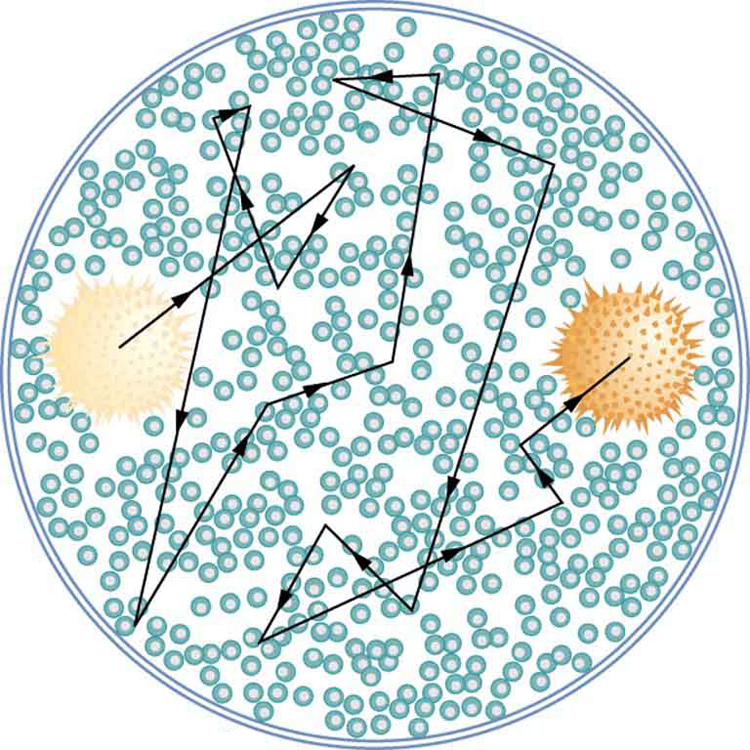
It was Albert Einstein who, starting in his epochal year of 1905, published several papers that explained precisely how Brownian motion could be used to measure the size of atoms and molecules. (In 1905 Einstein created special relativity, proposed photons as quanta of EM radiation, and produced a theory of Brownian motion that allowed the size of atoms to be determined. All of this was done in his spare time, since he worked days as a patent examiner. Any one of these very basic works could have been the crowning achievement of an entire career—yet Einstein did even more in later years.) Their sizes were only approximately known to be , based on a comparison of latent heat of vaporization and surface tension made in about 1805 by Thomas Young of double-slit fame and the famous astronomer and mathematician Simon Laplace.
Using Einstein’s ideas, the French physicist Jean-Baptiste Perrin (1870–1942) carefully observed Brownian motion; not only did he confirm Einstein’s theory, he also produced accurate sizes for atoms and molecules. Since molecular weights and densities of materials were well established, knowing atomic and molecular sizes allowed a precise value for Avogadro’s number to be obtained. (If we know how big an atom is, we know how many fit into a certain volume.) Perrin also used these ideas to explain atomic and molecular agitation effects in sedimentation, and he received the 1926 Nobel Prize for his achievements. Most scientists were already convinced of the existence of atoms, but the accurate observation and analysis of Brownian motion was conclusive—it was the first truly direct evidence.
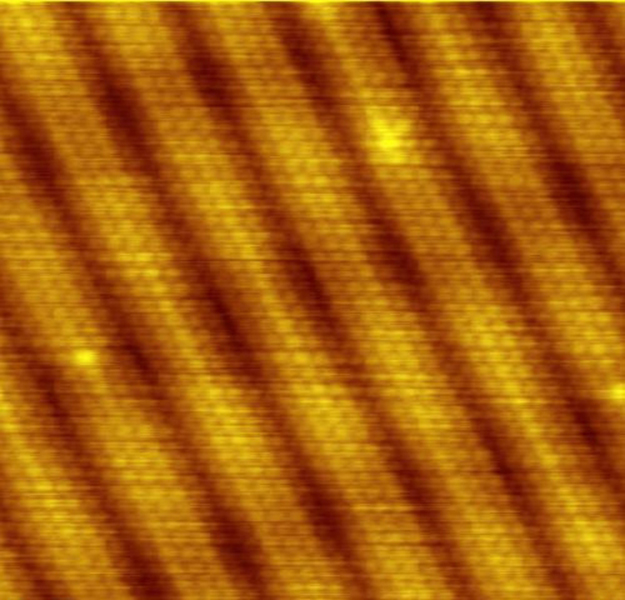
A huge array of direct and indirect evidence for the existence of atoms now exists. For example, it has become possible to accelerate ions (much as electrons are accelerated in cathode-ray tubes) and to detect them individually as well as measure their masses (see More Applications of Magnetism for a discussion of mass spectrometers). Other devices that observe individual atoms, such as the scanning tunneling electron microscope, will be discussed elsewhere. (See [link] .) All of our understanding of the properties of matter is based on and consistent with the atom. The atom’s substructures, such as electron shells and the nucleus, are both interesting and important. The nucleus in turn has a substructure, as do the particles of which it is composed. These topics, and the question of whether there is a smallest basic structure to matter, will be explored in later parts of the text.
Name three different types of evidence for the existence of atoms.
Explain why patterns observed in the periodic table of the elements are evidence for the existence of atoms, and why Brownian motion is a more direct type of evidence for their existence.
If atoms exist, why can’t we see them with visible light?
Using the given charge-to-mass ratios for electrons and protons, and knowing the magnitudes of their charges are equal, what is the ratio of the proton’s mass to the electron’s? (Note that since the charge-to-mass ratios are given to only three-digit accuracy, your answer may differ from the accepted ratio in the fourth digit.)
(a) Calculate the mass of a proton using the charge-to-mass ratio given for it in this chapter and its known charge. (b) How does your result compare with the proton mass given in this chapter?
If someone wanted to build a scale model of the atom with a nucleus 1.00 m in diameter, how far away would the nearest electron need to be?
50 km

Notification Switch
Would you like to follow the 'College physics' conversation and receive update notifications?