| << Chapter < Page | Chapter >> Page > |
The equations for the dissolution are:
Mercury(II) sulfide dissolves in a solution of sodium sulfide because HgS reacts with the S 2– ion:
A complex ion consists of a central atom, typically a transition metal cation, surrounded by ions, or molecules called ligands . These ligands can be neutral molecules like H 2 O or NH 3 , or ions such as CN – or OH – . Often, the ligands act as Lewis bases, donating a pair of electrons to the central atom. The ligands aggregate themselves around the central atom, creating a new ion with a charge equal to the sum of the charges and, most often, a transitional metal ion. This more complex arrangement is why the resulting ion is called a complex ion . The complex ion formed in these reactions cannot be predicted; it must be determined experimentally. The types of bonds formed in complex ions are called coordinate covalent bonds, as electrons from the ligands are being shared with the central atom. Because of this, complex ions are sometimes referred to as coordination complexes. This will be studied further in upcoming chapters.
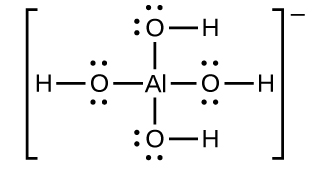
The equilibrium constant for the reaction of the components of a complex ion to form the complex ion in solution is called a formation constant ( K f ) (sometimes called a stability constant). For example, the complex ion is shown here:
It forms by the reaction:
At equilibrium:
The inverse of the formation constant is the dissociation constant ( K d ) , the equilibrium constant for the decomposition of a complex ion into its components in solution. We will work with dissociation constants further in the exercises for this section. Appendix K and [link] are tables of formation constants. In general, the larger the formation constant, the more stable the complex; however, as in the case of K sp values, the stoichiometry of the compound must be considered.
| Common Complex Ions by Decreasing Formulation Constants | |
|---|---|
| Substance | K f at 25 °C |
| 3 10 18 | |
| 1.7 10 7 | |
| 7 10 19 |
As an example of dissolution by complex ion formation, let us consider what happens when we add aqueous ammonia to a mixture of silver chloride and water. Silver chloride dissolves slightly in water, giving a small concentration of Ag + ([Ag + ] = 1.3 10 –5 M ):
However, if NH 3 is present in the water, the complex ion, can form according to the equation:
with
The large size of this formation constant indicates that most of the free silver ions produced by the dissolution of AgCl combine with NH 3 to form As a consequence, the concentration of silver ions, [Ag + ], is reduced, and the reaction quotient for the dissolution of silver chloride, [Ag + ][Cl – ], falls below the solubility product of AgCl:
More silver chloride then dissolves. If the concentration of ammonia is great enough, all of the silver chloride dissolves.

Notification Switch
Would you like to follow the 'Ut austin - principles of chemistry' conversation and receive update notifications?