| << Chapter < Page | Chapter >> Page > |
It is the destiny of wine to be drunk, and it is the destiny of glucose to be oxidized. But it was not oxidized immediately: its drinker kept it in his liver for more than a week, well curled up and tranquil, as a reserve aliment for a sudden effort; an effort that he was forced to make the following Sunday, pursuing a bolting horse.Primo Levi, The Periodic Table , 1975
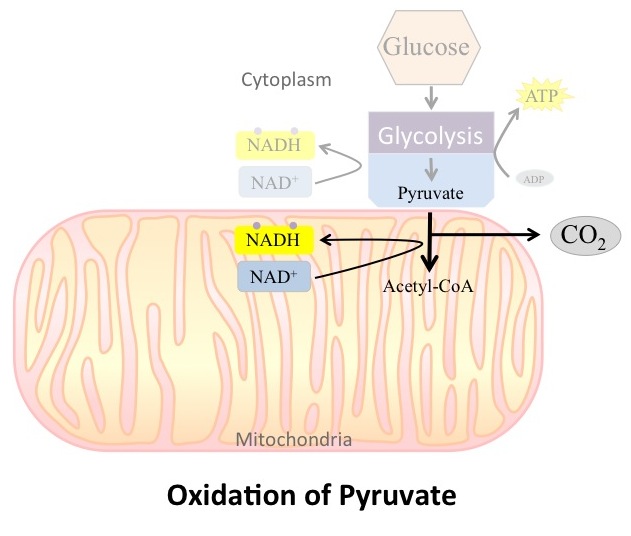
If oxygen is available, aerobic cellular respiration will go forward. In eukaryotic cells, the pyruvate molecules produced at the end of glycolysis are transported into mitochondria, which are the sites of aerobic cellular respiration. There, pyruvate (three carbons) will be transformed into an acetyl group (two carbons) that will be attached to a carrier compound called coenzyme A (CoA). The resulting compound is called acetyl-CoA . CoA is made from vitamin B5, pantothenic acid. Acetyl-CoA can be used in a variety of ways by the cell, but its major function is to deliver the two-carbon energy source derived from pyruvate to the next stage of the aerobic cellular respiration pathway.
In order for pyruvate, the product of glycolysis, to enter the next pathway, it must undergo several changes. The conversion is a three-step process ( [link] ).
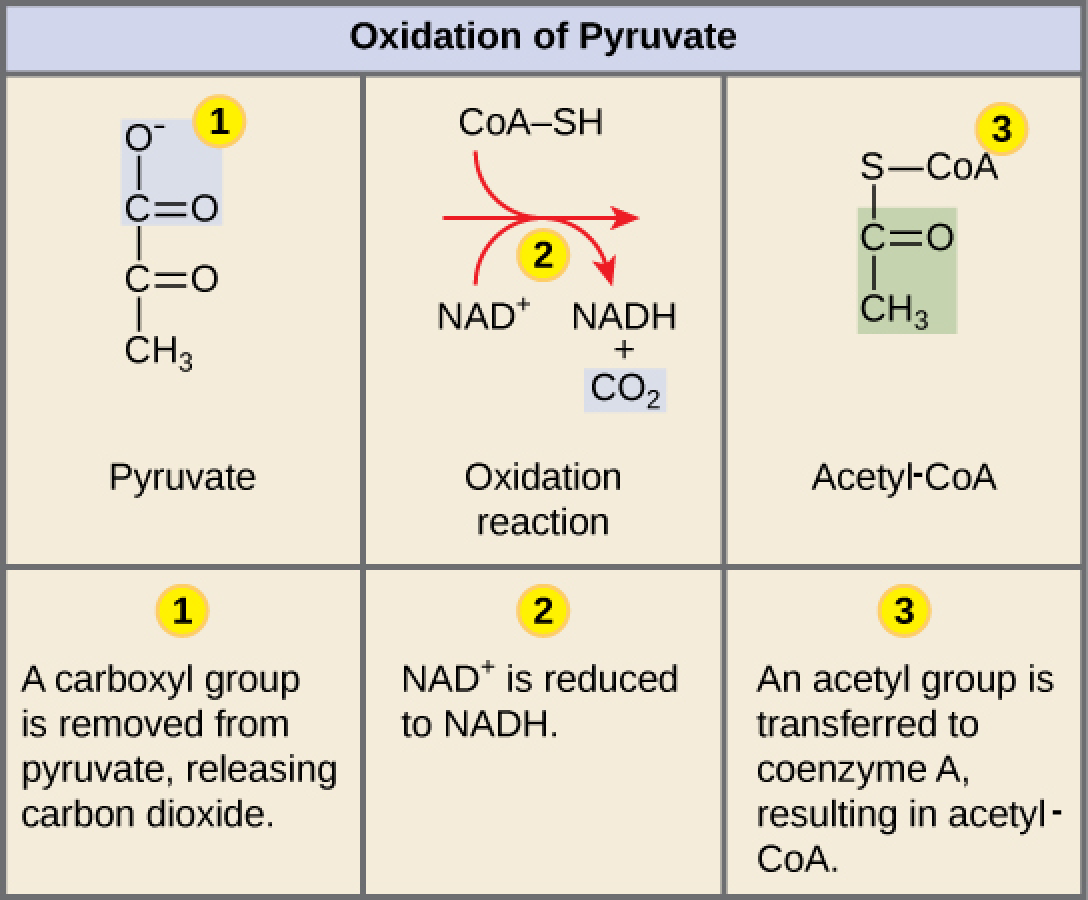
Step 1. A carboxyl group is removed from pyruvate, releasing a molecule of carbon dioxide into the surrounding medium. The result of this step is a two-carbon hydroxyethyl group bound to the enzyme (pyruvate dehydrogenase). This is the first of the six carbons from the original glucose molecule to be removed. This step proceeds twice for each glucose molecule (remember: there are two pyruvate molecules produced at the end of glycolsis). Thus, two of the six carbons will have been removed at the end of this step in aerobic cellular respiration.
Step 2. The hydroxyethyl group is oxidized to an acetyl group, and the electrons are picked up by NAD + , forming NADH. The high-energy electrons from NADH will be used later to generate ATP.
Step 3. The enzyme-bound acetyl group is transferred to CoA, producing a molecule of acetyl-CoA.
During the oxidation of pyruvate, the incoming pyruvate is converted to acetyl-CoA, NAD
+ is reduced to NADH and a carbon dioxide is released for each pyruvate entering the stage (
[link] ).
In the presence of oxygen, the two carbons in acetyl-CoA are added to to a four-carbon molecule, oxaloacetate, to form citrate (aka citric acid), a six-carbon molecule. This is the starting point of the Krebs Cycle, which will harvest the remainder of the extractable energy from what began as a glucose molecule. This single pathway has several different names: the citric acid cycle (for citric acid, the first compound in the cycle), the tricarboxylic acid (TCA) cycle (since citric acid has 3 carboxyl groups, and is thus a tricarboxylic acids), and the Krebs cycle , after Hans Krebs, who first identified the steps in the pathway in the 1930s in pigeon flight muscles. This work earned Krebs a share (with Fritz Lippman) of the 1953 Nobel Prize in Physiology and Medicine.

Notification Switch
Would you like to follow the 'Principles of biology' conversation and receive update notifications?