| << Chapter < Page | Chapter >> Page > |

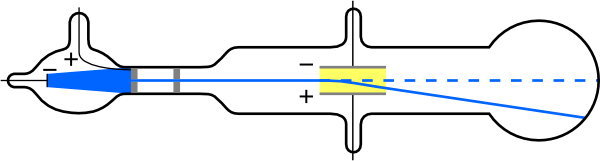
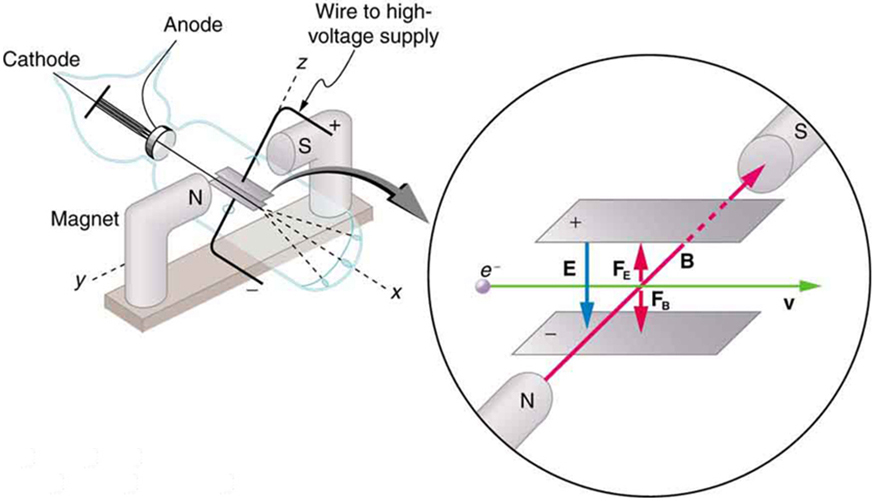
What is so important about , the ratio of the electron’s charge to its mass? The value obtained is
This is a huge number, as Thomson realized, and it implies that the electron has a very small mass. Thomson went on to do the same experiment for positively charged hydrogen ions (now known to be bare protons) and found a charge per kilogram about 1000 times smaller than that for the electron, implying that the proton is about 1000 times more massive than the electron. Today, we know more precisely that
where is the charge of the proton and is its mass. This ratio (to four significant figures) is 1836 times less charge per kilogram than for the electron. Since the charges of electrons and protons are equal in magnitude, this implies .
Thomson performed a variety of experiments using differing gases in discharge tubes and employing other methods, such as the photoelectric effect, for freeing electrons from atoms. He always found the same properties for the electron, proving it to be an independent particle. For his work, the important pieces of which he began to publish in 1897, Thomson was awarded the 1906 Nobel Prize in Physics. In retrospect, it is difficult to appreciate how astonishing it was to find that the atom has a substructure. Thomson himself said, “It was only when I was convinced that the experiment left no escape from it that I published my belief in the existence of bodies smaller than atoms.”
Thomson attempted to measure the charge of individual electrons, but his method could determine its charge only to the order of magnitude expected.
An American physicist, Robert Millikan (1868–1953) (see [link] ), decided to improve upon Thomson’s experiment for measuring and was eventually forced to try another approach, which is now a classic experiment performed by students. The Millikan oil drop experiment is shown in [link] .

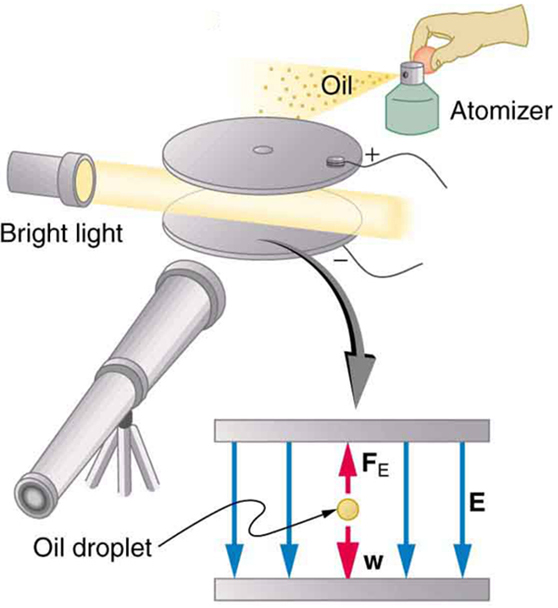
By 1913 Millikan had measured the charge of the electron to an accuracy of 1%, and he improved this by a factor of 10 within a few years to a value of . He also observed that all charges were multiples of the basic electron charge and that sudden changes could occur in which electrons were added or removed from the drops. For this very fundamental direct measurement of and for his studies of the photoelectric effect, Millikan was awarded the 1923 Nobel Prize in Physics.

Notification Switch
Would you like to follow the 'Concepts of physics' conversation and receive update notifications?