| << Chapter < Page | Chapter >> Page > |
We have observed competing processes in dynamic equilibrium in several different types of chemical processes. These include evaporation in dynamic liquid-vapor equilibrium with condensation. Similarly, we observed liquid-solid equilibrium, solutions in equilibrium, and solubility equilibrium involving both gases and ionic solids. It is clear from all of these studies that we need to understand the rates at which different processes occur, including what factors determine these rates. So far, these processes almost all involve pretty simple dynamics with fairly easy-to -understand rates. As we expand our studies into chemical reactions, however, we will encounter more complicated processes with more complicated rates of reaction.
It is important to understand rates of chemical reactions for many reasons, only one of which is understanding equilibrium. In many cases, the speed of the reaction might be of more interest than the final equilibrium conditions of the reaction. Some reactions proceed so slowly towards equilibrium as to appear to not occur at all. For example, metallic iron will eventually oxidize in the presence of aqueous salt solutions, but the time it takes for this process to occur is sufficiently long that we can reasonably expect to build a boat out of iron. On the other hand, some reactions may be so rapid as to pose a hazard. For example, hydrogen gas will react with oxygen gas so rapidly as to cause an explosion. In addition, the time scale for a reaction can depend very strongly on the amounts of reactants and their temperature.
In this Concept Development Study, we seek an understanding of the rates of chemical reactions. We will define and measure reaction rates and develop a quantitative analysis of the dependence of the reaction rates on the conditions of the reaction, including reactant concentrations and temperature. In this study, we will restrict ourselves to this quantitative analysis of reaction rates. In the following study, we will use this insight to develop a model to provide an understanding of the significance of reactant concentration and temperature.
In this study, we will assume very little prior knowledge, as we are beginning a new area of observation. We of course assume our understanding of the Atomic Molecular Theory, including the ideas of stoichiometry and balanced chemical equations. We will also assume an understanding of the postulates of the Kinetic Molecular Theory and of the energetics of chemical reactions.
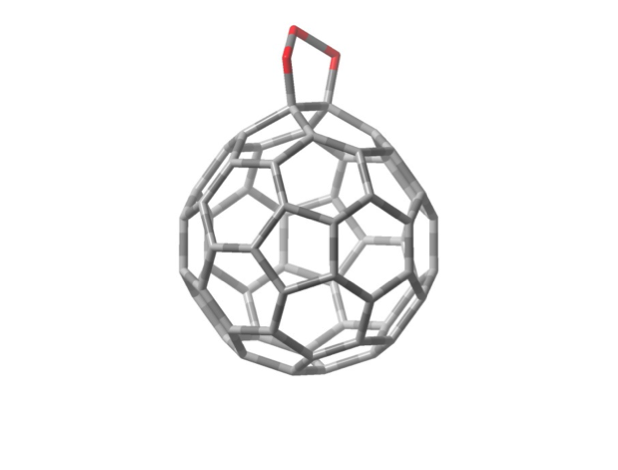
C 60 O 3 is prepared from C 60 dissolved in toluene solution at temperatures of 0 ºC or below. When the solution is warmed, C 60 O 3 decomposes, releasing O 2 and creating C 60 O. This reaction goes essentially to completion, in other words all of the C 60 O 3 decomposes creating an equal number of moles of C 60 O. We can actually watch this process happen in time by measuring the amount of light of a specific frequency absorbed by the C 60 O 3 molecules, called the “absorbance.” The absorbance is proportional to the concentration of the C 60 O 3 in the toluene solution, so observing the absorbance as a function of time is essentially the same as observing the concentration as a function of time. One such set of data is given in Table 1, which is shown in the graph in Figure 2.

Notification Switch
Would you like to follow the 'Concept development studies in chemistry 2013' conversation and receive update notifications?