| << Chapter < Page | Chapter >> Page > |
To spot the difference between these two molecules, we need to rely on our previously developed knowledge of electronegativities, molecular geometries, and molecular polarity. HCl is a polar linear molecular because the Cl atom is much more electronegative than the H atom. Si atoms are actually slightly less electronegative than H atoms, suggesting that SiH 4 should also be polar. But, we also recall from our electron domain model that SiH 4 has a symmetric tetrahedral geometry, like CH 4 . As such, like CH 4 , SiH 4 has no molecular dipole moment. From this comparison, we can conclude that, in comparing two molecules with similar dispersion forces, the molecule with a dipole moment will have stronger intermolecular attractions than the molecule without a dipole moment. This explains why, in each period in Figure 5, the Group IV hydride compound always has the lowest boiling point. Each of these compounds has nonpolar molecules due to their symmetry. By contrast, two HCl molecules will have stronger attractions as the positive end of one HCl will be attracted to the negative end of the other HCl, and vice versa.
We can now conclude that molecules attract one another via dispersion forces and, if the molecules are polar, via dipole-dipole attractions. Understanding these two types of intermolecular attractions works well to explain the major patterns observed in Figure 5.
This does not explain the exceptions, however. Why are the boiling points of NH 3 , H 2 O, and HF so abnormally high? Given that these are small mass molecules, we would not expect them to have larger than average dispersion forces. All three are polar molecules, but there is nothing to suggest that there dipole moments are unusually high. There must be a different type of intermolecular attraction that is unique to these three molecules out of this set of molecules.
We need a pattern to analyze. What do these three molecules have in common with each other that the other molecules in Table 1 do not? N, O, and F are all strongly electronegative atoms and are also amongst the smallest atoms in the periodic table. In the Lewis structures for all three molecules, O, N and F all have non-bonded, lone pairs of electrons. These three properties, taken together in a single molecule, must present a uniquely strong intermolecular attraction.
Chemists account for this strong bonding via a model called “hydrogen bonding.” This is a uniquely strong form of dipole-dipole attraction that only occurs when a molecule contains a hydrogen atom bonded to an N atom, an O atom, or an F atom. Due to the strength of the electronegativity of these atoms, the N-H bond or O-H bond or F-H bond is highly polar, meaning that the H atom is almost a bare, positively charged hydrogen nucleus. This strong positive charge on one molecule is in turn strongly attracted to the negatively charged lone pair electrons on the N, O, or F atom of another molecule. This is illustrated in Figure 7.
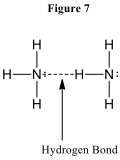
Note that size seems to be important in hydrogen bonding as well. HF has a much higher boiling point than HCl, indicating that HF has stronger intermolecular attractions. Even though the Cl atom is strongly electronegative and has lone pair electrons in the HCl molecule, the Cl atom is apparently too large to support the uniquely strong dipole-dipole attraction we call hydrogen bonding.

Notification Switch
Would you like to follow the 'Concept development studies in chemistry 2013' conversation and receive update notifications?