| << Chapter < Page | Chapter >> Page > |
The problems given in the exams are like those on exercises and lab work. The students who spend more time and effort in doing exercises and lab assignments will certainly have better performance in the exams. Exam scores are given in the range from 0 to 10.
Lecture Notes, Exercises, Laboratory Manual
Available at the instructor’s website.
Text book:
[1] G. J. Bronson, Program Development and Design Using C++, 3nd Edition, Brooks/COLE Thomson Learning, 2006.
(All lecture notes and exercises used in this course are mainly from this textbook. Students can find more C++ language features, problem-solving guidelines as well as good exercises and assignments from this book for further self-study.)
Reference books:
[2] H. M Deitel and P. J. Deitel, C++ How to Program – 3rd Edition, Prentice-Hall, 2001.
(This book contains a lot of good C++ programming examples. All examples in this book are very well explained.)
[3] J. Farrel, Object-Oriented Programming Using C++, 2nd Edition, Course Technology/Thomson Learning, 2001.
(If students want to go in depth in object-oriented programming with C++, they will find this book very helpful.)
[4] D. Gosselin, Microsoft Visual C++ 6.0, Course Technology/Thomson Learning, 2001.
(This book is a supplementary text for the students who want to know more about Microsoft Visual C++ 6.0 programming environment.)
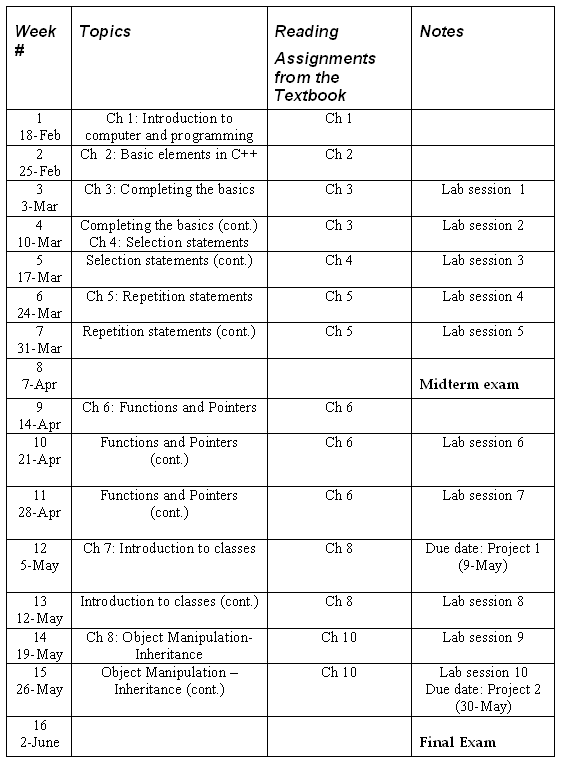
There are 16 sessions which compose of 14 lectures, 1 mid-term exam and 1 final exam.
To continuously improve course content and design, students are requested to complete end-of-course evaluation form. The student comments will be carefully reviewed and used to improve the quality of the course. Please take a moment to complete the following end-of-course feedback form.
OVERALL
______Very Satisfied
______Satisfied
______Neutral
______Dissatisfied
______Very Dissatisfied
______Very Satisfied
______Satisfied
______Neutral
______Dissatisfied
______Very Dissatisfied
----------------------------------------------------------------------------------------------
----------------------------------------------------------------------------------------------
COURSE CONTENT
______Very Satisfied
______Satisfied
______Neutral
______Dissatisfied
______Very Dissatisfied
______Very Satisfied
______Satisfied
______Neutral
______Dissatisfied
______Very Dissatisfied
______Very Satisfied
______Satisfied
______Neutral
______Dissatisfied
______Very Dissatisfied
______Very Satisfied
______Satisfied
______Neutral
______Dissatisfied
______Very Dissatisfied
------------------------------------------------------------------------------------------
------------------------------------------------------------------------------------------
INSTRUCTION
______Very Satisfied
______Satisfied
______Neutral
______Dissatisfied
______Very Dissatisfied
______Very Satisfied
______Satisfied
______Neutral
______Dissatisfied
______Very Dissatisfied
--------------------------------------------------------------------------------------------
--------------------------------------------------------------------------------------------
FACILITIES AND EQUIPMENT
______Very Satisfied
______Satisfied
______Neutral
______Dissatisfied
______Very Dissatisfied
______Very Satisfied
______Satisfied
______Neutral
______Dissatisfied
______Very Dissatisfied
------------------------------------------------------------------------------------------------
------------------------------------------------------------------------------------------------
SUPPORT
______Very Satisfied
______Satisfied
______Neutral
______Dissatisfied
______Very Dissatisfied
------------------------------------------------------------------------------------------------
------------------------------------------------------------------------------------------------
Thank you for your comments.

Notification Switch
Would you like to follow the 'Programming fundamentals in c++' conversation and receive update notifications?