| << Chapter < Page | Chapter >> Page > |
At 1600 °C, quartz melts to yield a viscous liquid. When the liquid cools, it does not crystallize readily but usually supercools and forms a glass, also called silica. The SiO 4 tetrahedra in glassy silica have a random arrangement characteristic of supercooled liquids, and the glass has some very useful properties. Silica is highly transparent to both visible and ultraviolet light. For this reason, it is important in the manufacture of lamps that give radiation rich in ultraviolet light and in certain optical instruments that operate with ultraviolet light. The coefficient of expansion of silica glass is very low; therefore, rapid temperature changes do not cause it to fracture. CorningWare and other ceramic cookware contain amorphous silica.
Silicates are salts containing anions composed of silicon and oxygen. In nearly all silicates, sp 3 -hybridized silicon atoms occur at the centers of tetrahedra with oxygen at the corners. There is a variation in the silicon-to-oxygen ratio that occurs because silicon-oxygen tetrahedra may exist as discrete, independent units or may share oxygen atoms at corners in a variety of ways. In addition, the presence of a variety of cations gives rise to the large number of silicate minerals.
Many ceramics are composed of silicates. By including small amounts of other compounds, it is possible to modify the physical properties of the silicate materials to produce ceramics with useful characteristics.
The elements boron, silicon, germanium, arsenic, antimony, and tellurium separate the metals from the nonmetals in the periodic table. These elements, called metalloids or sometimes semimetals, exhibit properties characteristic of both metals and nonmetals. The structures of these elements are similar in many ways to those of nonmetals, but the elements are electrical semiconductors.
Give the hybridization of the metalloid and the molecular geometry for each of the following compounds or ions. You may wish to review the chapters on chemical bonding and advanced covalent bonding for relevant examples.
(a) GeH 4
(b) SbF 3
(c) Te(OH) 6
(d) H 2 Te
(e) GeF 2
(f) TeCl 4
(g)
(h) SbCl 5
(i) TeF 6
Write a Lewis structure for each of the following molecules or ions. You may wish to review the chapter on chemical bonding.
(a) H 3 BPH 3
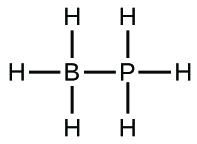
(b)
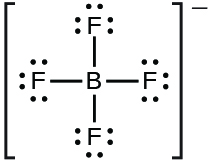
(c) BBr 3
(d) B(CH 3 ) 3
(e) B(OH) 3
(a) H
3 BPH
3 :
 ;
;
(b)
 ;
;
(c) BBr
3 :
 ;
;
(d) B(CH
3 )
3 :
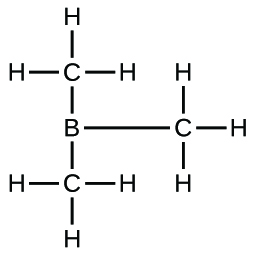 ;
;
(e) B(OH)
3 :
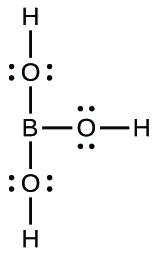
Describe the hybridization of boron and the molecular structure about the boron in each of the following:
(a) H 3 BPH 3
(b)
(c) BBr 3
(d) B(CH 3 ) 3
(e) B(OH) 3
Using only the periodic table, write the complete electron configuration for silicon, including any empty orbitals in the valence shell. You may wish to review the chapter on electronic structure.
1 s 2 2 s 2 2 p 6 3 s 2 3 p 2 3 d 0 .
Write a Lewis structure for each of the following molecules and ions:
(a) (CH 3 ) 3 SiH
(b)
(c) Si 2 H 6
(d) Si(OH) 4
(e)
Describe the hybridization of silicon and the molecular structure of the following molecules and ions:
(a) (CH 3 ) 3 SiH
(b)
(c) Si 2 H 6
(d) Si(OH) 4
(e)
(a) (CH 3 ) 3 SiH: sp 3 bonding about Si; the structure is tetrahedral; (b) sp 3 bonding about Si; the structure is tetrahedral; (c) Si 2 H 6 : sp 3 bonding about each Si; the structure is linear along the Si-Si bond; (d) Si(OH) 4 : sp 3 bonding about Si; the structure is tetrahedral; (e) sp 3 d 2 bonding about Si; the structure is octahedral
Describe the hybridization and the bonding of a silicon atom in elemental silicon.
Classify each of the following molecules as polar or nonpolar. You may wish to review the chapter on chemical bonding.
(a) SiH 4
(b) Si 2 H 6
(c) SiCl 3 H
(d) SiF 4
(e) SiCl 2 F 2
(a) nonpolar; (b) nonpolar; (c) polar; (d) nonpolar; (e) polar
Silicon reacts with sulfur at elevated temperatures. If 0.0923 g of silicon reacts with sulfur to give 0.3030 g of silicon sulfide, determine the empirical formula of silicon sulfide.
Name each of the following compounds:
(a) TeO 2
(b) Sb 2 S 3
(c) GeF 4
(d) SiH 4
(e) GeH 4
(a) tellurium dioxide or tellurium(IV) oxide; (b) antimony(III) sulfide; (c) germanium(IV) fluoride; (d) silane or silicon(IV) hydride; (e) germanium(IV) hydride
Write a balanced equation for the reaction of elemental boron with each of the following (most of these reactions require high temperature):
(a) F 2
(b) O 2
(c) S
(d) Se
(e) Br 2
Why is boron limited to a maximum coordination number of four in its compounds?
Boron has only s and p orbitals available, which can accommodate a maximum of four electron pairs. Unlike silicon, no d orbitals are available in boron.
Write a formula for each of the following compounds:
(a) silicon dioxide
(b) silicon tetraiodide
(c) silane
(d) silicon carbide
(e) magnesium silicide
From the data given in Appendix I , determine the standard enthalpy change and the standard free energy change for each of the following reactions:
(a)
(b)
(c)
(a) Δ H ° = 87 kJ; Δ G ° = 44 kJ; (b) Δ H ° = −109.9 kJ; Δ G° = −154.7 kJ; (c) Δ H ° = −510 kJ; Δ G ° = −601.5 kJ
A hydride of silicon prepared by the reaction of Mg 2 Si with acid exerted a pressure of 306 torr at 26 °C in a bulb with a volume of 57.0 mL. If the mass of the hydride was 0.0861 g, what is its molecular mass? What is the molecular formula for the hydride?
Suppose you discovered a diamond completely encased in a silicate rock. How would you chemically free the diamond without harming it?
A mild solution of hydrofluoric acid would dissolve the silicate and would not harm the diamond.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?