| << Chapter < Page | Chapter >> Page > |
The structures of the nonmetals differ dramatically from those of metals. Metals crystallize in closely packed arrays that do not contain molecules or covalent bonds. Nonmetal structures contain covalent bonds, and many nonmetals consist of individual molecules. The electrons in nonmetals are localized in covalent bonds, whereas in a metal, there is delocalization of the electrons throughout the solid.
The noble gases are all monatomic, whereas the other nonmetal gases—hydrogen, nitrogen, oxygen, fluorine, and chlorine—normally exist as the diatomic molecules H 2, N 2 , O 2 , F 2 , and Cl 2 . The other halogens are also diatomic; Br 2 is a liquid and I 2 exists as a solid under normal conditions. The changes in state as one moves down the halogen family offer excellent examples of the increasing strength of intermolecular London forces with increasing molecular mass and increasing polarizability.
Oxygen has two allotropes: O 2 , dioxygen, and O 3 , ozone. Phosphorus has three common allotropes, commonly referred to by their colors: white, red, and black. Sulfur has several allotropes. There are also many carbon allotropes. Most people know of diamond, graphite, and charcoal, but fewer people know of the recent discovery of fullerenes, carbon nanotubes, and graphene.
Descriptions of the physical properties of three nonmetals that are characteristic of molecular solids follow.
Carbon occurs in the uncombined (elemental) state in many forms, such as diamond, graphite, charcoal, coke, carbon black, graphene, and fullerene.
Diamond, shown in [link] , is a very hard crystalline material that is colorless and transparent when pure. Each atom forms four single bonds to four other atoms at the corners of a tetrahedron ( sp 3 hybridization); this makes the diamond a giant molecule. Carbon-carbon single bonds are very strong, and, because they extend throughout the crystal to form a three-dimensional network, the crystals are very hard and have high melting points (~4400 °C).
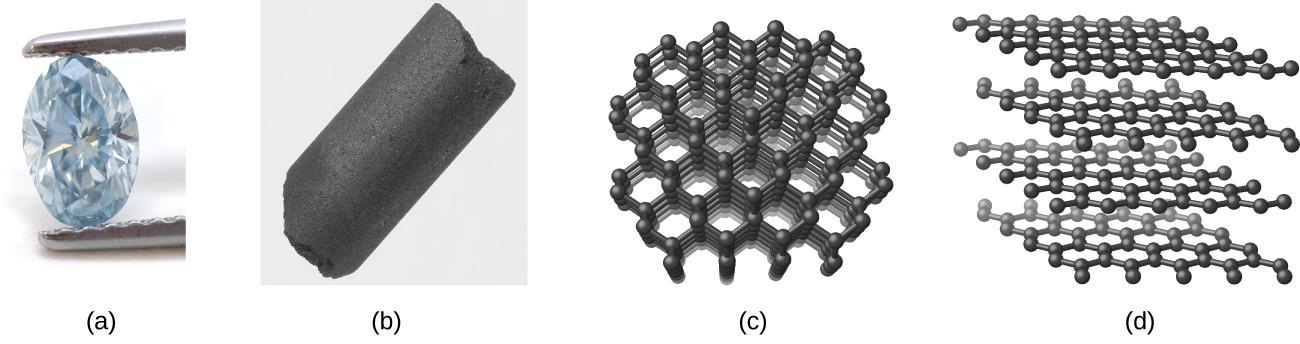
Graphite, also shown in [link] , is a soft, slippery, grayish-black solid that conducts electricity. These properties relate to its structure, which consists of layers of carbon atoms, with each atom surrounded by three other carbon atoms in a trigonal planar arrangement. Each carbon atom in graphite forms three σ bonds, one to each of its nearest neighbors, by means of sp 2 -hybrid orbitals. The unhybridized p orbital on each carbon atom will overlap unhybridized orbitals on adjacent carbon atoms in the same layer to form π bonds. Many resonance forms are necessary to describe the electronic structure of a graphite layer; [link] illustrates two of these forms.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?