| << Chapter < Page | Chapter >> Page > |
As we discuss osmotic pressure in blood and tissue fluid, it is important to recognize that the formed elements of blood do not contribute to osmotic concentration gradients. Rather, it is the plasma proteins that play the key role. Solutes also move across the capillary wall according to their concentration gradient, but overall, the concentrations should be similar and not have a significant impact on osmosis. Because of their large size and chemical structure, plasma proteins are not truly solutes, that is, they do not dissolve but are dispersed or suspended in their fluid medium, forming a colloid rather than a solution.
The pressure created by the concentration of colloidal proteins in the blood is called the blood colloidal osmotic pressure (BCOP) . Its effect on capillary exchange accounts for the reabsorption of water. The plasma proteins suspended in blood cannot move across the semipermeable capillary cell membrane, and so they remain in the plasma. As a result, blood has a higher colloidal concentration and lower water concentration than tissue fluid. It therefore attracts water. We can also say that the BCOP is higher than the interstitial fluid colloidal osmotic pressure (IFCOP) , which is always very low because interstitial fluid contains few proteins. Thus, water is drawn from the tissue fluid back into the capillary, carrying dissolved molecules with it. This difference in colloidal osmotic pressure accounts for reabsorption.
The normal unit used to express pressures within the cardiovascular system is millimeters of mercury (mm Hg). When blood leaving an arteriole first enters a capillary bed, the CHP is quite high—about 35 mm Hg. Gradually, this initial CHP declines as the blood moves through the capillary so that by the time the blood has reached the venous end, the CHP has dropped to approximately 18 mm Hg. In comparison, the plasma proteins remain suspended in the blood, so the BCOP remains fairly constant at about 25 mm Hg throughout the length of the capillary and considerably below the osmotic pressure in the interstitial fluid.
The net filtration pressure (NFP) represents the interaction of the hydrostatic and osmotic pressures, driving fluid out of the capillary. It is equal to the difference between the CHP and the BCOP. Since filtration is, by definition, the movement of fluid out of the capillary, when reabsorption is occurring, the NFP is a negative number.
NFP changes at different points in a capillary bed ( [link] ). Close to the arterial end of the capillary, it is approximately 10 mm Hg, because the CHP of 35 mm Hg minus the BCOP of 25 mm Hg equals 10 mm Hg. Recall that the hydrostatic and osmotic pressures of the interstitial fluid are essentially negligible. Thus, the NFP of 10 mm Hg drives a net movement of fluid out of the capillary at the arterial end. At approximately the middle of the capillary, the CHP is about the same as the BCOP of 25 mm Hg, so the NFP drops to zero. At this point, there is no net change of volume: Fluid moves out of the capillary at the same rate as it moves into the capillary. Near the venous end of the capillary, the CHP has dwindled to about 18 mm Hg due to loss of fluid. Because the BCOP remains steady at 25 mm Hg, water is drawn into the capillary, that is, reabsorption occurs. Another way of expressing this is to say that at the venous end of the capillary, there is an NFP of −7 mm Hg.
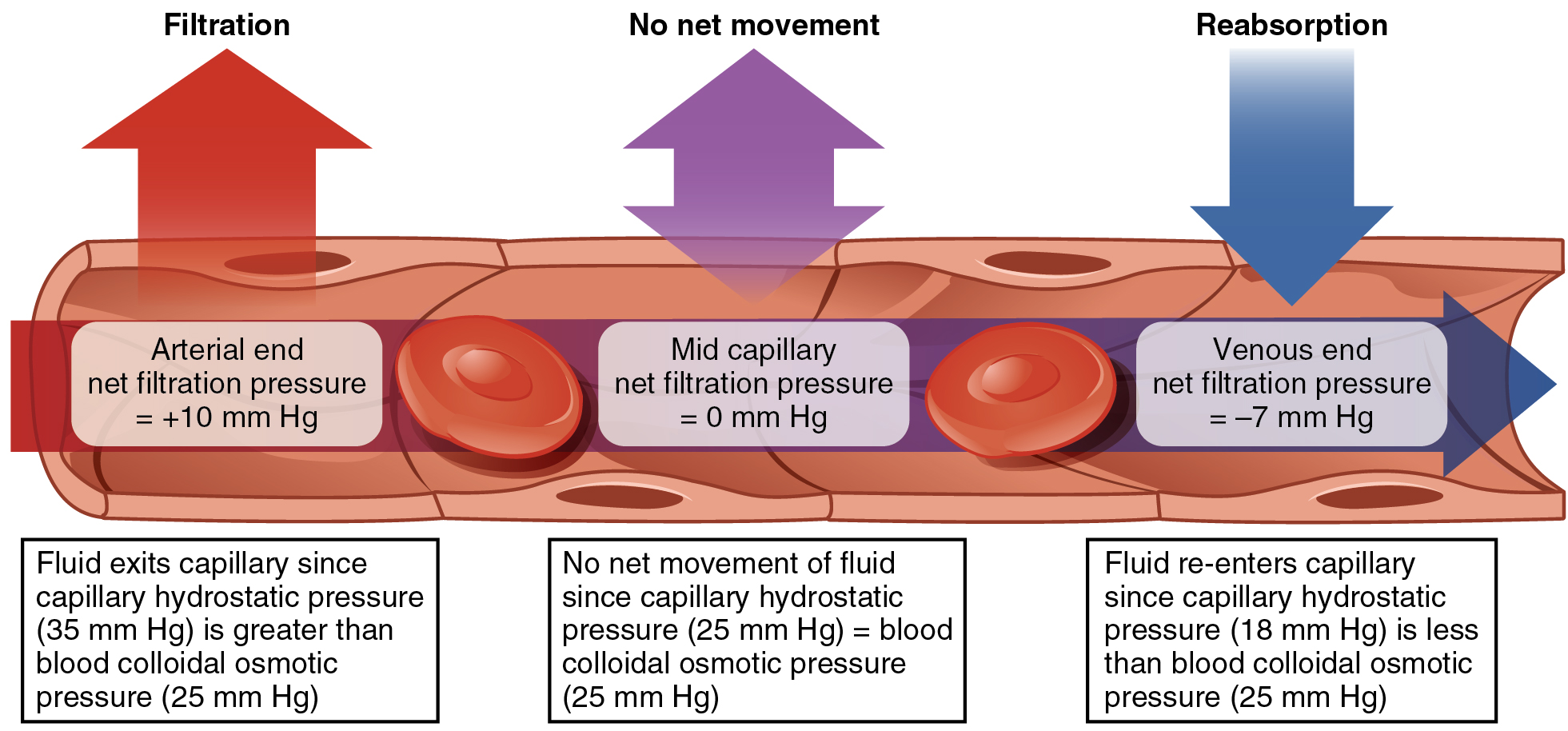
Since overall CHP is higher than BCOP, it is inevitable that more net fluid will exit the capillary through filtration at the arterial end than enters through reabsorption at the venous end. Considering all capillaries over the course of a day, this can be quite a substantial amount of fluid: Approximately 24 liters per day are filtered, whereas 20.4 liters are reabsorbed. This excess fluid is picked up by capillaries of the lymphatic system. These extremely thin-walled vessels have copious numbers of valves that ensure unidirectional flow through ever-larger lymphatic vessels that eventually drain into the subclavian veins in the neck. An important function of the lymphatic system is to return the fluid (lymph) to the blood. Lymph may be thought of as recycled blood plasma . (Seek additional content for more detail on the lymphatic system.)
Watch this video to explore capillaries and how they function in the body. Capillaries are never more than 100 micrometers away. What is the main component of interstitial fluid?
Small molecules can cross into and out of capillaries via simple or facilitated diffusion. Some large molecules can cross in vesicles or through clefts, fenestrations, or gaps between cells in capillary walls. However, the bulk flow of capillary and tissue fluid occurs via filtration and reabsorption. Filtration, the movement of fluid out of the capillaries, is driven by the CHP. Reabsorption, the influx of tissue fluid into the capillaries, is driven by the BCOP. Filtration predominates in the arterial end of the capillary; in the middle section, the opposing pressures are virtually identical so there is no net exchange, whereas reabsorption predominates at the venule end of the capillary. The hydrostatic and colloid osmotic pressures in the interstitial fluid are negligible in healthy circumstances.
Watch this video to explore capillaries and how they function in the body. Capillaries are never more than 100 micrometers away. What is the main component of interstitial fluid?
Water.

Notification Switch
Would you like to follow the 'Anatomy & Physiology' conversation and receive update notifications?