| << Chapter < Page | Chapter >> Page > |
Just as interesting as the effects of heat transfer on a system are the methods by which it occurs. Whenever there is a temperature difference, heat transfer occurs. It may occur rapidly, as through a cooking pan, or slowly, as through the walls of a picnic ice chest. So many processes involve heat transfer that it is hard to imagine a situation where no heat transfer occurs. Yet every heat transfer takes place by only three methods:
In the illustration at the beginning of this chapter, the fire warms the snowshoers’ faces largely by radiation. Convection carries some heat to them, but most of the air flow from the fire is upward (creating the familiar shape of flames), carrying heat to the food being cooked and into the sky. The snowshoers wear clothes designed with low conductivity to prevent heat flow out of their bodies.
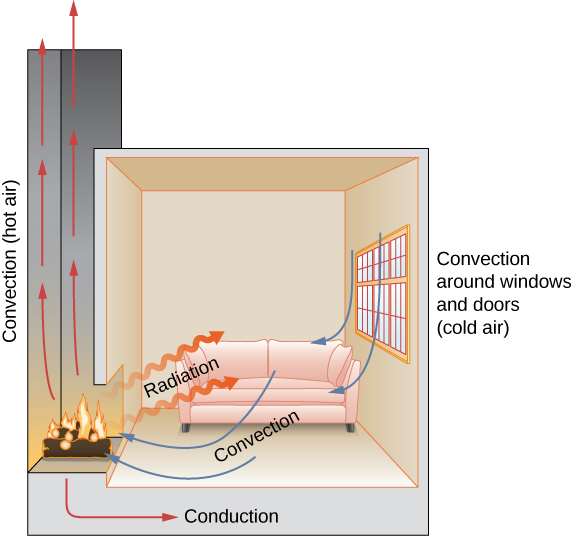
In this section, we examine these methods in some detail. Each method has unique and interesting characteristics, but all three have two things in common: They transfer heat solely because of a temperature difference, and the greater the temperature difference, the faster the heat transfer ( [link] ).
Check Your Understanding Name an example from daily life (different from the text) for each mechanism of heat transfer.
Conduction: Heat transfers into your hands as you hold a hot cup of coffee. Convection: Heat transfers as the barista “steams” cold milk to make hot cocoa. Radiation: Heat transfers from the Sun to a jar of water with tea leaves in it to make “Sun tea.” A great many other answers are possible.

Notification Switch
Would you like to follow the 'University physics volume 2' conversation and receive update notifications?