| << Chapter < Page | Chapter >> Page > |
A closed system is one in which energy can enter or leave, but matter cannot. The second beaker covered by the bell jar is an example of a closed system. The beaker can still be heated or cooled, but water vapour cannot leave the system because the bell jar is a barrier. Condensation changes the vapour to liquid and returns it to the beaker. In other words, there is no loss of matter from the system.
An open system is one whose borders allow the movement of energy and matter into and out of the system. A closed system is one in which only energy can be exchanged, but not matter.
Some reactions can take place in two directions. In one direction the reactants combine to form the products. This is called the forward reaction . In the other, the products react to form reactants again. This is called the reverse reaction . A special double-headed arrow is used to show this type of reversible reaction :
So, in the following reversible reaction:
The forward reaction is . The reverse reaction is .
A reversible reaction is a chemical reaction that can proceed in both the forward and reverse directions. In other words, the reactant and product of one reaction may reverse roles.
Apparatus and materials:
Lime water (Ca(OH) ); calcium carbonate (CaCO ); hydrochloric acid; 2 test tubes with rubber stoppers; delivery tube; retort stand and clamp; bunsen burner.
Method and observations:
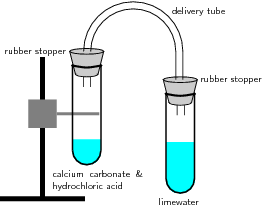
Discussion:
Using the same reversible reaction that we used in an earlier example:
The forward reaction is:
The reverse reaction is:
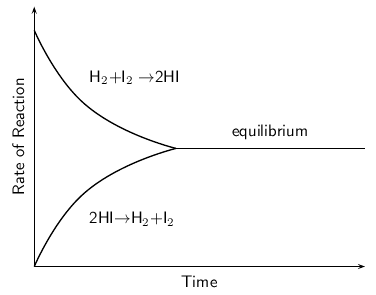
When the rate of the forward reaction and the reverse reaction are equal, the system is said to be in equilbrium . [link] shows this. Initially (time = 0), the rate of the forward reaction is high and the rate of the reverse reaction is low. As the reaction proceeds, the rate of the forward reaction decreases and the rate of the reverse reaction increases, until both occur at the same rate. This is called equilibrium.
Although it is not always possible to observe any macroscopic changes, this does not mean that the reaction has stopped. The forward and reverse reactions continue to take place and so microscopic changes still occur in the system. This state is called dynamic equilibrium . In the liquid-vapour phase equilibrium demonstration, dynamic equilibrium was reached when there was no observable change in the level of the water in the second beaker even though evaporation and condensation continued to take place.
There are, however, a number of factors that can change the chemical equilibrium of a reaction. Changing the concentration , the temperature or the pressure of a reaction can affect equilibrium. These factors will be discussed in more detail later in this chapter.
Chemical equilibrium is the state of a chemical reaction, where the concentrations of the reactants and products have no net change over time. Usually, this state results when the forward chemical reactions proceed at the same rate as their reverse reactions.

Notification Switch
Would you like to follow the 'Siyavula textbooks: grade 12 physical science' conversation and receive update notifications?