| << Chapter < Page | Chapter >> Page > |
The shape of a covalent molecule can be predicted using the Valence Shell Electron Pair Repulsion (VSEPR) theory. This is a model in chemistry that tries to predict the shapes of molecules. Very simply, VSEPR theory says that the valence electron pairs in a molecule will arrange themselves around the central atom of the molecule so that the repulsion between their negative charges is as small as possible. In other words, the valence electron pairs arrange themselves so that they are as far apart as they can be. The number of valence electron pairs in the molecule determines the dhape of that molecule.
Valence shell electron pair repulsion (VSEPR) theory is a model in chemistry, which is used to predict the shape of individual molecules, based upon the extent of their electron-pair repulsion.
VSEPR theory is based on the idea that the geometry of a molecule is mostly determined by repulsion among the pairs of electrons around a central atom. The pairs of electrons may be bonding or non-bonding (also called lone pairs). Only valence electrons of the central atom influence the molecular shape in a meaningful way.
To predict the shape of a covalent molecule, follow these steps:
Step 1:
Draw the molecule using Lewis notation. Make sure that you draw all the electrons around the molecule's central atom.
Step 2:
Count the number of electron pairs around the central atom.
Step 3:
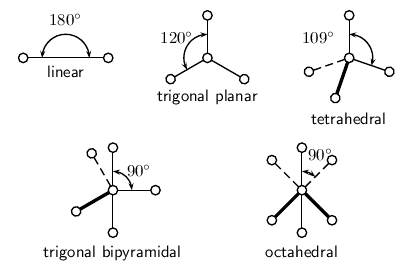
Determine the basic geometry of the molecule using the table below. For example, a molecule with two electron pairs around the central atom has a linear shape, and one with four electron pairs around the central atom would have a tetrahedral shape. The situation is actually more complicated than this, but this will be discussed later in this section.
| Number of electron pairs | Geometry |
| 2 | linear |
| 3 | trigonal planar |
| 4 | tetrahedral |
| 5 | trigonal bipyramidal |
| 6 | octahedral |
[link] shows each of these shapes. Remember that the shapes are 3-dimensional, and so you need to try to imagine them in this way. In the diagrams, the thicker lines represents those parts of the molecule that are 'in front' (or coming out of the page), while the dashed lines represent those parts that are 'at the back' (or going into the page) of the molecule.
The simulation in covalent bonding also allows you to view the molecules in 3-D. The shape of the molecules in this view is the shape of the molecule predicted by VSEPR.
You can also view different molecules and see their shapes at this website . You do not need to know all these molecules, this is simply to give you a feel for what molecules look like.
Determine the shape of a molecule of
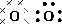
There are two electron pairs.
Since there are two electron pairs, the molecule must be linear.
Determine the shape of a molecule of
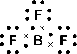
There are three electron pairs.
Since there are three electron pairs, the molecule must be trigonal planar.
Determining the shape of a molecule can be a bit more complicated. In the examples we have used above, we looked only at the number of bonding electron pairs when we were trying to decide on the molecules' shape. But there are also other electron pairs in the molecules. These electrons, which are not involved in bonding but which are also around the central atom, are called lone pairs . The worked example below will give you an indea of how these lone pairs can affect the shape of the molecule.
Determine the shape of a molecule of

There are four electron pairs.
Since there are four electron pairs, the molecule must be tetrahedral.
There is one lone pair of electrons and this will affect the shape of the molecule.
The lone pair needs more space than the bonding pairs, and therefore pushes the three hydrogen atoms together a little more. The bond angles between the hydrogen and nitrogen atoms in the molecule become 106 degrees, rather than the usual 109 degrees of a tetrahedral molecule. The shape of the molecule is trigonal pyramidal .
In groups, you are going to build a number of molecules using marshmallows to represent the atoms in the molecule, and toothpicks to represent the bonds between the atoms. In other words, the toothpicks will hold the atoms (marshmallows) in the molecule together. Try to use different coloured marshmallows to represent different elements.
You will build models of the following molecules:
HCl, , , HBr and NH
For each molecule, you need to:
Do the models help you to have a clearer picture of what the molecules look like? Try to build some more models for other molecules you can think of.

Notification Switch
Would you like to follow the 'Siyavula textbooks: grade 11 physical science' conversation and receive update notifications?