| << Chapter < Page | Chapter >> Page > |

If you consider a very small object such as a grain of pollen, in a gas, then the number of atoms and molecules striking its surface would also be relatively small. Would the grain of pollen experience any fluctuations in pressure due to statistical fluctuations in the number of gas atoms and molecules striking it in a given amount of time?
Yes. Such fluctuations actually occur for a body of any size in a gas, but since the numbers of atoms and molecules are immense for macroscopic bodies, the fluctuations are a tiny percentage of the number of collisions, and the averages spoken of in this section vary imperceptibly. Roughly speaking the fluctuations are proportional to the inverse square root of the number of collisions, so for small bodies they can become significant. This was actually observed in the 19th century for pollen grains in water, and is known as the Brownian effect.
Pump gas molecules into a box and see what happens as you change the volume, add or remove heat, change gravity, and more. Measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other.

Two samples of ideal gas in separate containers have the same number of molecules and the same temperature, but the molecular mass of gas X is greater than that of gas Y. Which of the following correctly compares the average speed of the molecules of the gases and the average force the gases exert on their respective containers?
| Average Speed of Molecules | Average Force on Container | |
| (a) | Greater for gas X | Greater for gas X |
| (b) | Greater for gas X | The forces cannot be compared without knowing the volumes of the gases. |
| (c) | Greater for gas Y | Greater for gas Y |
| (d) | Greater for gas Y | The forces cannot be compared without knowing the volumes of the gases. |
(d)
How will the average kinetic energy of a gas molecule change if its temperature is increased from 20ºC to 313ºC?
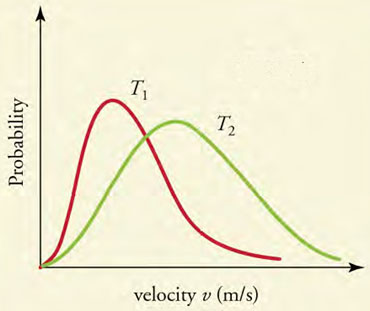
This graph shows the Maxwell-Boltzmann distribution of molecular speeds in an ideal gas for two temperatures, T 1 and T 2 . Which of the following statements is false?
(b)
Suppose you have gas in a cylinder with a movable piston which has an area of 0.40 m 2 . The pressure of the gas is 150 Pa when the height of the piston is 0.02 m. Find the force exerted by the gas on the piston. How does this force change if the piston is moved to a height of 0.03 m? Assume temperature remains constant.

Notification Switch
Would you like to follow the 'College physics for ap® courses' conversation and receive update notifications?