| << Chapter < Page | Chapter >> Page > |
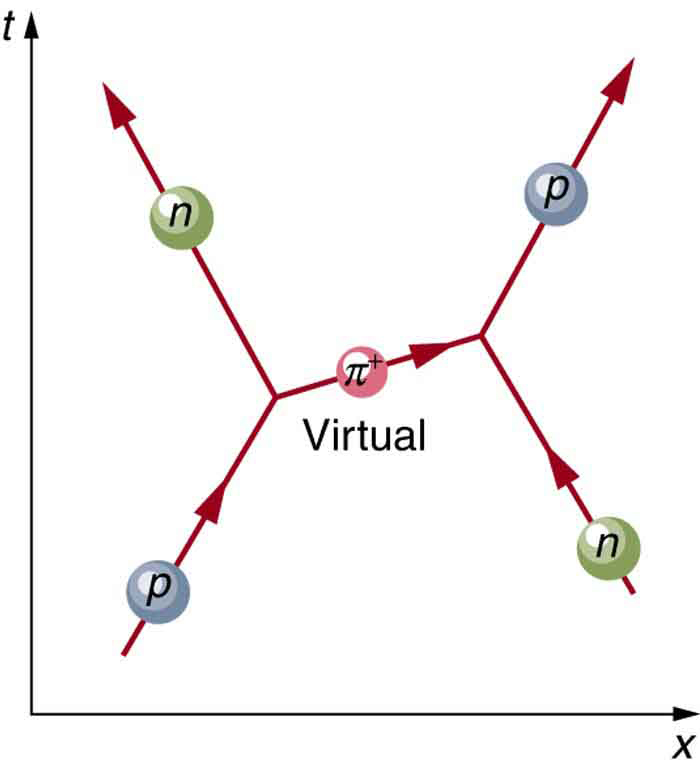
The relative strengths of the forces given in the [link] are those for the most common situations. When particles are brought very close together, the relative strengths change, and they may become identical at extremely close range. As we shall see in GUTs: the Unification of Forces , carrier particles may be altered by the energy required to bring particles very close together—in such a manner that they become identical.
You are familiar with gravity pulling you towards the Earth. It's why when you jump, you come back down. In this action, and at distances and speeds that we experience in our everyday lives, gravity is the only one of the four fundamental forces that has such an obvious effect on us.
Electromagnetism is vital for our society to run, but due to your body having the same (or very nearly the same) number of positive and negative charges, it doesn't usually have as much of an effect on us. Except for one very important feature: the electrons in the bottom of your feet experience a mutually repulsive force with the electrons in the material you stand on. This is what keeps us from falling into the planet, and also allows us to push on other objects and generally interact with them.
These electromagnetic forces are dominant in the electron shells of an atom, and also the interaction of the electrons with the nucleus. However, within the nucleus, the electrostatic repulsion of the protons would break the nucleus apart if it were not for the strong force, which holds the nucleus together. At even smaller scales, within nucleons such as protons and neutrons, the weak force is responsible for nuclear decays.
The relative strengths of the forces given in the [link] are those for the most common situations. When particles are brought very close together, the relative strengths change, and they may become identical at extremely close range. As we shall see in GUTs: the Unification of Forces , carrier particles may be altered by the energy required to bring particles very close together—in such a manner that they become identical.
Two intact (not ionized) hydrogen atoms are 10 cm apart. Which of the following are true?
(d)
Explain why we only need to concern ourselves with gravitational force to describe the orbit of the Earth around the Sun.
Consider four forces: the gravitational force between the Earth and the Sun; the electrostatic force between the Earth and the Sun; the gravitational force between the proton and electron in a hydrogen atom, and the electrostatic force between the proton and electron in a hydrogen atom. What is the proper ordering of the magnitude of these forces, from greatest to least?
(d)
Deep within a nucleon, which is the stronger force between two quarks, gravity or the weak force? Why do you think so?
Consider the Earth-Moon system. If we were to place equal charges on the Earth and the Moon, how large would they need to be for the electrostatic repulsion to counteract the gravitational attraction?
(b)
What is the strength of the magnetic field created by the orbiting Moon, at the center of the orbit, in the system in the previous problem? (Treat the charge going around in orbit as a current loop.) How does this compare with the strength of the Earth's intrinsic magnetic field?
An atomic nucleus consists of positively charged protons and neutral neutrons, so the electrostatic repulsion should destroy it by making the protons fly apart. This doesn't happen because:
(a)
The atomic number of an atom is the number of protons in that atom's nucleus. Make a prediction as to what happens to electromagnetic repulsion as the atomic number gets larger. Then, make a further prediction about what this implies about the number of neutrons in heavy nuclei.
(a) Find the ratio of the strengths of the weak and electromagnetic forces under ordinary circumstances.
(b) What does that ratio become under circumstances in which the forces are unified?
(a) to 1, weak to EM
(b) 1 to 1
The ratio of the strong to the weak force and the ratio of the strong force to the electromagnetic force become 1 under circumstances where they are unified. What are the ratios of the strong force to those two forces under normal circumstances?

Notification Switch
Would you like to follow the 'College physics for ap® courses' conversation and receive update notifications?