| << Chapter < Page | Chapter >> Page > |
Ionisation energy is the energy that is needed to remove one electron from an atom. The ionisation energy will be different for different atoms.
The second ionisation energy is the energy that is needed to remove a second electron from an atom, and so on. As an energy level becomes more full, it becomes more and more difficult to remove an electron and the ionisation energy increases . On the Periodic Table of the Elements, a group is a vertical column of the elements, and a period is a horizontal row. In the periodic table, ionisation energy increases across a period, but decreases as you move down a group. The lower the ionisation energy, the more reactive the element will be because there is a greater chance of electrons being involved in chemical reactions. We will look at this in more detail in the next section.
Match the information in column A with the information in column B by writing only the letter (A to I) next to the question number (1 to 7)
| 1. A positive ion that has 3 less electrons than its neutral atom | A. Mg |
| 2. An ion that has 1 more electron than its neutral atom | B. Cl |
| 3. The anion that is formed when bromine gains an electron | C. CO |
| 4. The cation that is formed from a magnesium atom | D. Al |
| 5. An example of a compound ion | E. Br |
| 6. A positive ion with the electron configuration of argon | F. K |
| 7. A negative ion with the electron configuration of neon | G. Mg |
| H. O | |
| I. Br |
The periodic table of the elements is a method of showing the chemical elements in a table. Most of the work that was done to arrive at the periodic table that we know, can be attributed to a man called Dmitri Mendeleev in 1869. Mendeleev was a Russian chemist who designed the table in such a way that recurring ("periodic") trends in the properties of the elements could be shown. Using the trends he observed, he even left gaps for those elements that he thought were 'missing'. He even predicted the properties that he thought the missing elements would have when they were discovered. Many of these elements were indeed discovered and Mendeleev's predictions were proved to be correct.
To show the recurring properties that he had observed, Mendeleev began new rows in his table so that elements with similar properties were in the same vertical columns, called groups . Each row was referred to as a period . One important feature to note in the periodic table is that all the non-metals are to the right of the zig-zag line drawn under the element boron. The rest of the elements are metals, with the exception of hydrogen which occurs in the first block of the table despite being a non-metal.
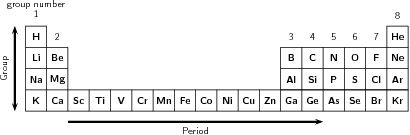
A group is a vertical column in the periodic table and is considered to be the most important way of classifying the elements. If you look at a periodic table, you will see the groups numbered at the top of each column. The groups are numbered from left to right as follows: 1, 2, then an open space which contains the transition elements , followed by groups 3 to 8. These numbers are normally represented using Roman numerals. In some periodic tables, all the groups are numbered from left to right from number 1 to number 18. In some groups, the elements display very similar chemical properties and the groups are even given separate names to identify them.

Notification Switch
Would you like to follow the 'Siyavula textbooks: grade 10 physical science' conversation and receive update notifications?