| << Chapter < Page | Chapter >> Page > |
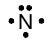
Note that this structure leaves three of the valence electrons “unpaired” and thus ready to join in a shared electron pair. The remaining two valence electrons are “paired,” and this notation implies that they therefore are not generally available for sharing in a covalent bond. This notation is consistent with the available data, i.e. five valence electrons and a valence of 3. Pairing the two non-bonding electrons seems reasonable in analogy to the fact that electrons are paired in forming covalent bonds.
We can draw similar structures for oxygen and fluorine. The other halogens will have structures like F, since they have the same valence and the same number of valence electrons.
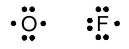
With this notation in hand, we can now analyze structures for molecules including nitrogen, oxygen, and the halogens. The hydrides are the easiest:
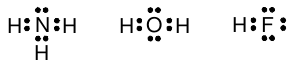
Note that the octet rule is clearly obeyed for oxygen, nitrogen, and the halogens.
At this point, it becomes very helpful to adopt one new convention: a pair of bonded electrons will now be more easily represented in our Lewis structures by a straight line, rather than two dots. Double bonds and triple bonds are respectively represented by double and triple straight lines between atoms. We will continue to show non-bonded electron pairs explicitly with two dots.
For example, ethanol has the molecular formula C 2 H 6 O. The two carbon atoms are bonded together and the oxygen atom is attached to one of the two carbons; the hydrogen atoms are arranged to complete the valences of the carbon atoms and the oxygen atom:
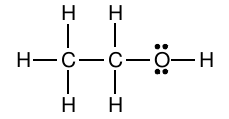
In this structure, each line connecting two atoms represents a shared pair of electrons, or a covalent bond. The non-bonding pairs on oxygen are often called “lone pairs.” It is important for us to include them in our structure for three reasons. First, including the lone pairs helps us check that we have drawn a structure with the correct number of valence electrons. Let’s check this for this drawing. Each carbon atom contributes four valence electrons, the oxygen atom contributes six, and each hydrogen atom contributes 1. There are thus a total of 2(4)+6+6(1)=20 valence electrons. Counting electrons in the drawing, there are eight covalent bonds, each of which represents two valence electrons, and two lone pairs, for a total of 20 valence electrons.
Second, drawing the lone pairs helps us see that the octet rule is obeyed for the O atom. Third and perhaps most importantly, we will later learn that lone pairs of electrons are important in determining the physical and chemical properties of molecules.

Notification Switch
Would you like to follow the 'Concept development studies in chemistry 2013' conversation and receive update notifications?