| << Chapter < Page | Chapter >> Page > |
| Number | Exponential Form | Common Logarithm |
|---|---|---|
| 1000 | 10 3 | 3 |
| 10 | 10 1 | 1 |
| 1 | 10 0 | 0 |
| 0.1 | 10 −1 | −1 |
| 0.001 | 10 −3 | −3 |
To find the common logarithm of most numbers, you will need to use the LOG button on a calculator.
In reporting numerical data obtained via measurements, we use only as many significant figures as the accuracy of the measurement warrants. For example, suppose a microbiologist using an automated cell counter determines that there are 525,341 bacterial cells in a one-liter sample of river water. However, she records the concentration as 525,000 cells per liter and uses this rounded number to estimate the number of cells that would likely be found in 10 liters of river water. In this instance, the last three digits of the measured quantity are not considered significant. They are rounded to account for variations in the number of cells that would likely occur if more samples were measured.
The importance of significant figures lies in their application to fundamental computation. In addition and subtraction, the sum or difference should contain as many digits to the right of the decimal as that in the least certain (indicated by underscoring in the following example) of the numbers used in the computation.
Suppose a microbiologist wishes to calculate the total mass of two samples of agar.
The least certain of the two masses has three decimal places, so the sum must have three decimal places.
In multiplication and division, the product or quotient should contain no more digits than than in the factor containing the least number of significant figures. Suppose the microbiologist would like to calculate how much of a reagent would be present in 6.6 mL if the concentration is 0.638 g/mL.
Again, the answer has only one decimal place because this is the accuracy of the least accurate number in the calculation.
When rounding numbers, increase the retained digit by 1 if it is followed by a number larger than 5 (“round up”). Do not change the retained digit if the digits that follow are less than 5 (“round down”). If the retained digit is followed by 5, round up if the retained digit is odd, or round down if it is even (after rounding, the retained digit will thus always be even).
It is possible to write an equation to calculate the cell numbers at any time if the number of starting cells and doubling time are known, as long as the cells are dividing at a constant rate. We define N 0 as the starting number of bacteria, the number at time t = 0. N i is the number of bacteria at time t = i , an arbitrary time in the future. Finally we will set j equal to the number of generations, or the number of times the cell population doubles during the time interval. Then we have,
This equation is an expression of growth by binary fission.
In our example, N 0 = 4, the number of generations, j , is equal to 3 after 90 minutes because the generation time is 30 minutes. The number of cells can be estimated from the following equation:
The number of cells after 90 minutes is 32.
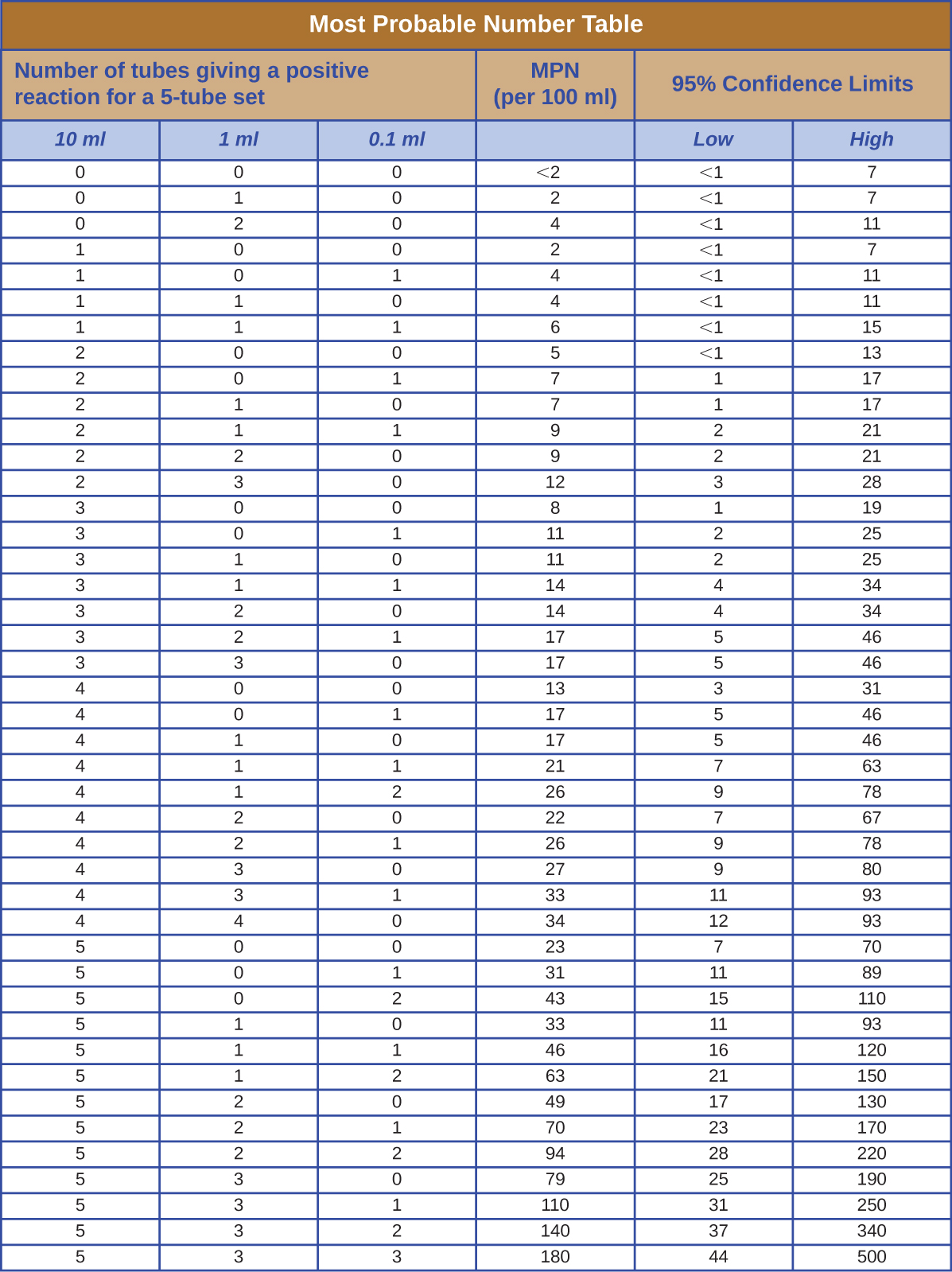
The table in [link] contains values used to calculate the most probable number example given in How Microbes Grow .

Notification Switch
Would you like to follow the 'Microbiology' conversation and receive update notifications?