| << Chapter < Page | Chapter >> Page > |
As the example implies, gravitational force is completely negligible on a small scale, where the interactions of individual charged particles are important. On a large scale, such as between the Earth and a person, the reverse is true. Most objects are nearly electrically neutral, and so attractive and repulsive Coulomb forces nearly cancel. Gravitational force on a large scale dominates interactions between large objects because it is always attractive, while Coulomb forces tend to cancel.
where and are two point charges separated by a distance , and
[link] shows the charge distribution in a water molecule, which is called a polar molecule because it has an inherent separation of charge. Given water’s polar character, explain what effect humidity has on removing excess charge from objects.
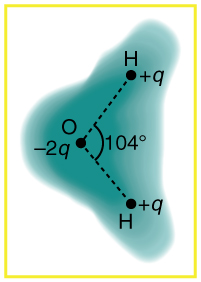
Given the polar character of water molecules, explain how ions in the air form nucleation centers for rain droplets.
What is the repulsive force between two pith balls that are 8.00 cm apart and have equal charges of – 30.0 nC?
(a) How strong is the attractive force between a glass rod with a charge and a silk cloth with a charge, which are 12.0 cm apart, using the approximation that they act like point charges? (b) Discuss how the answer to this problem might be affected if the charges are distributed over some area and do not act like point charges.
(a) 0.263 N
(b) If the charges are distributed over some area, there will be a concentration of charge along the side closest to the oppositely charged object. This effect will increase the net force.
Two point charges exert a 5.00 N force on each other. What will the force become if the distance between them is increased by a factor of three?
Two point charges are brought closer together, increasing the force between them by a factor of 25. By what factor was their separation decreased?
The separation decreased by a factor of 5.
How far apart must two point charges of 75.0 nC (typical of static electricity) be to have a force of 1.00 N between them?
If two equal charges each of 1 C each are separated in air by a distance of 1 km, what is the magnitude of the force acting between them? You will see that even at a distance as large as 1 km, the repulsive force is substantial because 1 C is a very significant amount of charge.
Bare free charges do not remain stationary when close together. To illustrate this, calculate the acceleration of two isolated protons separated by 2.00 nm (a typical distance between gas atoms).
(a) By what factor must you change the distance between two point charges to change the force between them by a factor of 10? (b) Explain how the distance can either increase or decrease by this factor and still cause a factor of 10 change in the force.
(a) 3.2
(b) If the distance increases by 3.2, then the force will decrease by a factor of 10 ; if the distance decreases by 3.2, then the force will increase by a factor of 10. Either way, the force changes by a factor of 10.
Suppose you have a total charge that you can split in any manner. Once split, the separation distance is fixed. How do you split the charge to achieve the greatest force?
(a) Common transparent tape becomes charged when pulled from a dispenser. If one piece is placed above another, the repulsive force can be great enough to support the top piece’s weight. Assuming equal point charges (only an approximation), calculate the magnitude of the charge if electrostatic force is great enough to support the weight of a 10.0 mg piece of tape held 1.00 cm above another. (b) Discuss whether the magnitude of this charge is consistent with what is typical of static electricity.
(a) C
(b) This charge is approximately 1 nC, which is consistent with the magnitude of charge typical for static electricity
(a) Find the ratio of the electrostatic to gravitational force between two electrons. (b) What is this ratio for two protons? (c) Why is the ratio different for electrons and protons?
At what distance is the electrostatic force between two protons equal to the weight of one proton?
A certain five cent coin contains 5.00 g of nickel. What fraction of the nickel atoms’ electrons, removed and placed 1.00 m above it, would support the weight of this coin? The atomic mass of nickel is 58.7, and each nickel atom contains 28 electrons and 28 protons.
(a) Two point charges totaling exert a repulsive force of 0.150 N on one another when separated by 0.500 m. What is the charge on each? (b) What is the charge on each if the force is attractive?

Notification Switch
Would you like to follow the 'Concepts of physics' conversation and receive update notifications?