| << Chapter < Page | Chapter >> Page > |
Cooking is essentially synthetic chemistry that happens to be safe to eat. There are a number of examples of acid-base chemistry in the culinary world. One example is the use of baking soda, or sodium bicarbonate in baking. NaHCO 3 is a base. When it reacts with an acid such as lemon juice, buttermilk, or sour cream in a batter, bubbles of carbon dioxide gas are formed from decomposition of the resulting carbonic acid, and the batter “rises.” Baking powder is a combination of sodium bicarbonate, and one or more acid salts that react when the two chemicals come in contact with water in the batter.
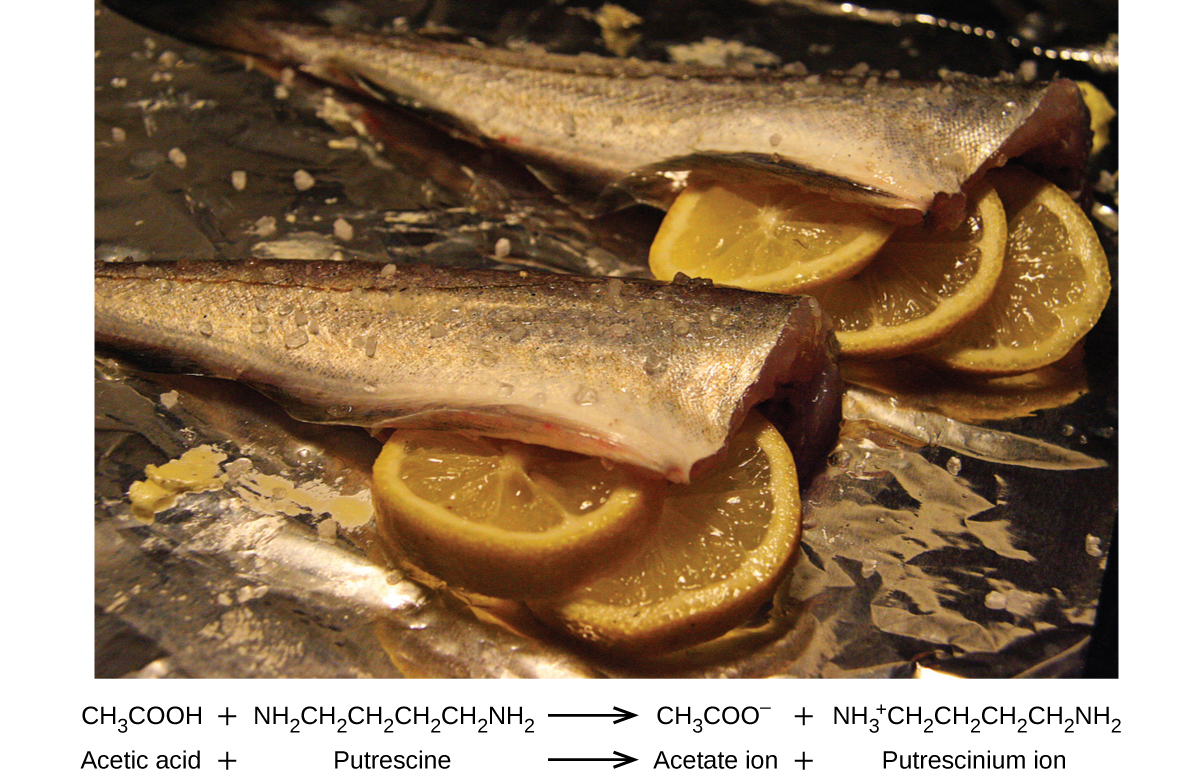
Many people like to put lemon juice or vinegar, both of which are acids, on cooked fish ( [link] ). It turns out that fish have volatile amines (bases) in their systems, which are neutralized by the acids to yield involatile ammonium salts. This reduces the odor of the fish, and also adds a “sour” taste that we seem to enjoy.
Pickling is a method used to preserve vegetables using a naturally produced acidic environment. The vegetable, such as a cucumber, is placed in a sealed jar submerged in a brine solution. The brine solution favors the growth of beneficial bacteria and suppresses the growth of harmful bacteria. The beneficial bacteria feed on starches in the cucumber and produce lactic acid as a waste product in a process called fermentation. The lactic acid eventually increases the acidity of the brine to a level that kills any harmful bacteria, which require a basic environment. Without the harmful bacteria consuming the cucumbers they are able to last much longer than if they were unprotected. A byproduct of the pickling process changes the flavor of the vegetables with the acid making them taste sour.
When we neutralize a weak base with a strong acid, the product is a salt containing the conjugate acid of the weak base. This conjugate acid is a weak acid. For example, ammonium chloride, NH 4 Cl, is a salt formed by the reaction of the weak base ammonia with the strong acid HCl:
A solution of this salt contains ammonium ions and chloride ions. The chloride ion has no effect on the acidity of the solution since HCl is a strong acid. Chloride is a very weak base and will not accept a proton to a measurable extent. However, the ammonium ion, the conjugate acid of ammonia, reacts with water and increases the hydronium ion concentration:
The equilibrium equation for this reaction is simply the ionization constant. K a , for the acid
We will not find a value of K a for the ammonium ion in Appendix H . However, it is not difficult to determine K a for from the value of the ionization constant of water, K w , and K b , the ionization constant of its conjugate base, NH 3 , using the following relationship:
This relation holds for any base and its conjugate acid or for any acid and its conjugate base.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?