| << Chapter < Page | Chapter >> Page > |
As we saw in the previous section, reactions proceed in both directions (reactants go to products and products go to reactants). We can tell a reaction is at equilibrium if the reaction quotient ( Q ) is equal to the equilibrium constant ( K ). We next address what happens when a system at equilibrium is disturbed so that Q is no longer equal to K . If a system at equilibrium is subjected to a perturbance or stress (such as a change in concentration) the position of equilibrium changes. Since this stress affects the concentrations of the reactants and the products, the value of Q will no longer equal the value of K . To re-establish equilibrium, the system will either shift toward the products (if Q< K) or the reactants (if Q> K ) until Q returns to the same value as K .
This process is described by Le Châtelier's principle : When a chemical system at equilibrium is disturbed, it returns to equilibrium by counteracting the disturbance. As described in the previous paragraph, the disturbance causes a change in Q ; the reaction will shift to re-establish Q = K .
Le Châtelier's principle can be used to predict changes in equilibrium concentrations when a system that is at equilibrium is subjected to a stress. However, if we have a mixture of reactants and products that have not yet reached equilibrium, the changes necessary to reach equilibrium may not be so obvious. In such a case, we can compare the values of Q and K for the system to predict the changes.
A chemical system at equilibrium can be temporarily shifted out of equilibrium by adding or removing one or more of the reactants or products. The concentrations of both reactants and products then undergo additional changes to return the system to equilibrium.
The stress on the system in [link] is the reduction of the equilibrium concentration of SCN − (lowering the concentration of one of the reactants would cause Q to be larger than K ). As a consequence, Le Châtelier's principle leads us to predict that the concentration of Fe(SCN) 2+ should decrease, increasing the concentration of SCN − part way back to its original concentration, and increasing the concentration of Fe 3+ above its initial equilibrium concentration.
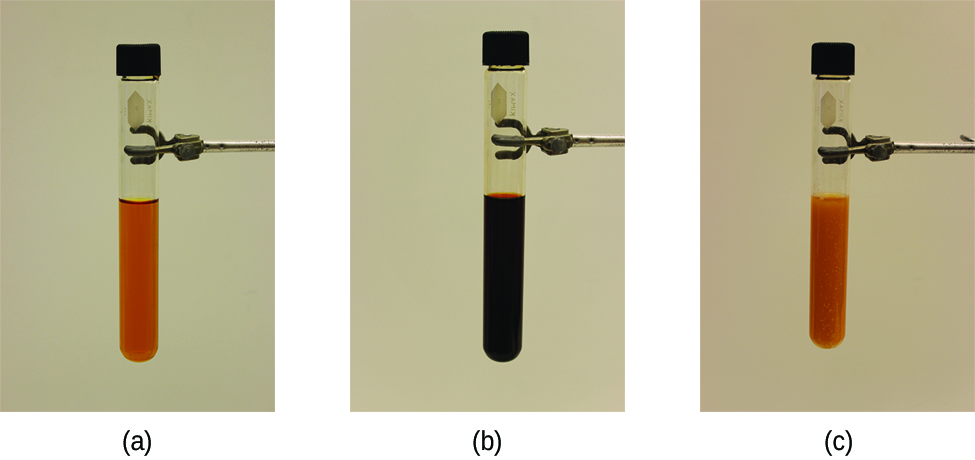
The effect of a change in concentration on a system at equilibrium is illustrated further by the equilibrium of this chemical reaction:

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?