| << Chapter < Page | Chapter >> Page > |
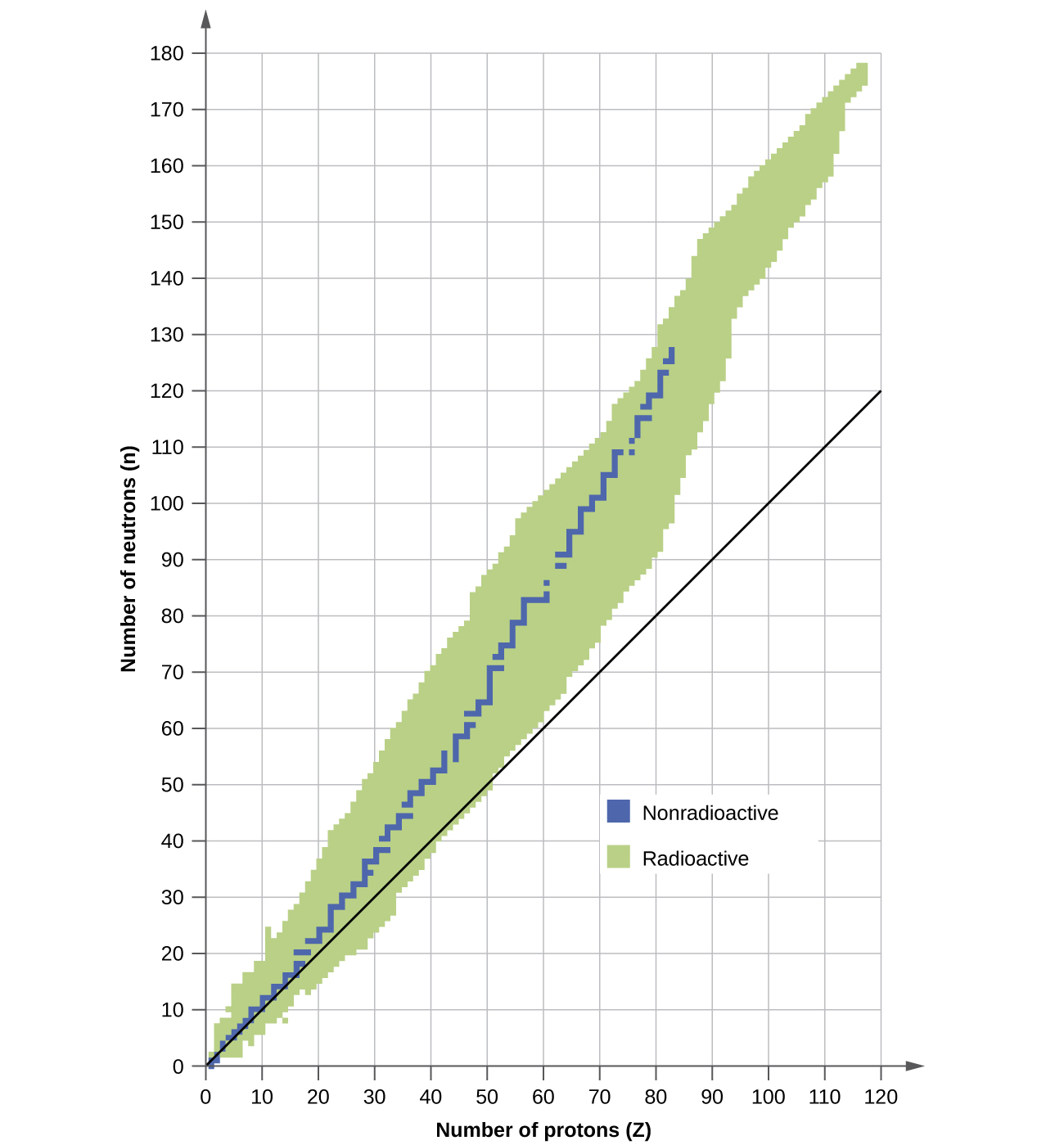
The nuclei that are to the left or to the right of the band of stability are unstable and exhibit radioactivity . They change spontaneously (decay) into other nuclei that are either in, or closer to, the band of stability. These nuclear decay reactions convert one unstable isotope (or radioisotope ) into another, more stable, isotope. We will discuss the nature and products of this radioactive decay in subsequent sections of this chapter.
Several observations may be made regarding the relationship between the stability of a nucleus and its structure. Nuclei with even numbers of protons, neutrons, or both are more likely to be stable (see [link] ). Nuclei with certain numbers of nucleons, known as magic numbers , are stable against nuclear decay. These numbers of protons or neutrons (2, 8, 20, 28, 50, 82, and 126) make complete shells in the nucleus. These are similar in concept to the stable electron shells observed for the noble gases. Nuclei that have magic numbers of both protons and neutrons, such as and are called “double magic” and are particularly stable. These trends in nuclear stability may be rationalized by considering a quantum mechanical model of nuclear energy states analogous to that used to describe electronic states earlier in this textbook. The details of this model are beyond the scope of this chapter.
| Stable Nuclear Isotopes | ||
|---|---|---|
| Number of Stable Isotopes | Proton Number | Neutron Number |
| 157 | even | even |
| 53 | even | odd |
| 50 | odd | even |
| 5 | odd | odd |
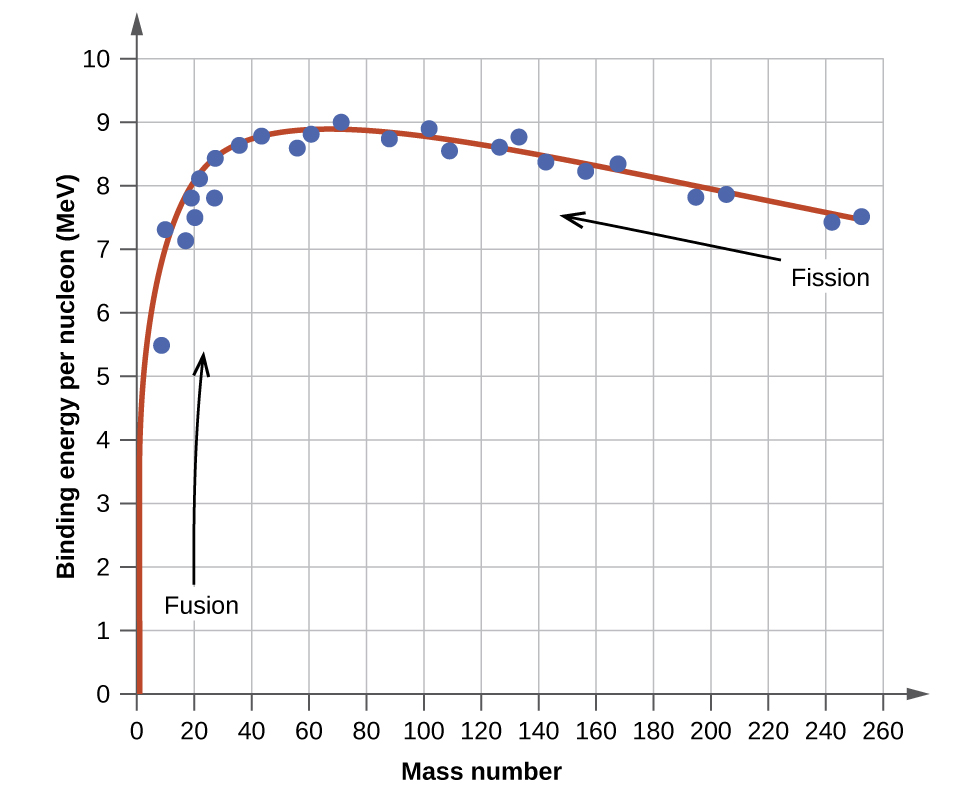
The relative stability of a nucleus is correlated with its binding energy per nucleon , the total binding energy for the nucleus divided by the number or nucleons in the nucleus. For instance, we saw in [link] that the binding energy for a nucleus is 28.4 MeV. The binding energy per nucleon for a nucleus is therefore:
In [link] , we learn how to calculate the binding energy per nucleon of a nuclide on the curve shown in [link] .
We next calculate the binding energy for one nucleus from the mass defect using the mass-energy equivalence equation:
We then convert the binding energy in joules per nucleus into units of MeV per nuclide:
Finally, we determine the binding energy per nucleon by dividing the total nuclear binding energy by the number of nucleons in the atom:
Note that this is almost 25% larger than the binding energy per nucleon for
(Note also that this is the same process as in [link] , but with the additional step of dividing the total nuclear binding energy by the number of nucleons.)
7.810 MeV/nucleon
An atomic nucleus consists of protons and neutrons, collectively called nucleons. Although protons repel each other, the nucleus is held tightly together by a short-range, but very strong, force called the strong nuclear force. A nucleus has less mass than the total mass of its constituent nucleons. This “missing” mass is the mass defect, which has been converted into the binding energy that holds the nucleus together according to Einstein’s mass-energy equivalence equation, E = mc 2 . Of the many nuclides that exist, only a small number are stable. Nuclides with even numbers of protons or neutrons, or those with magic numbers of nucleons, are especially likely to be stable. These stable nuclides occupy a narrow band of stability on a graph of number of protons versus number of neutrons. The binding energy per nucleon is largest for the elements with mass numbers near 56; these are the most stable nuclei.
Write the following isotopes in hyphenated form (e.g., “carbon-14”)
(a)
(b)
(c)
(d)
(a) sodium-24; (b) aluminum-29; (c) krypton-73; (d) iridium-194
Write the following isotopes in nuclide notation (e.g.,
(a) oxygen-14
(b) copper-70
(c) tantalum-175
(d) francium-217
For the following isotopes that have missing information, fill in the missing information to complete the notation
(a)
(b)
(c)
(d)
(a) (b) (c) (d)
For each of the isotopes in [link] , determine the numbers of protons, neutrons, and electrons in a neutral atom of the isotope.
Write the nuclide notation, including charge if applicable, for atoms with the following characteristics:
(a) 25 protons, 20 neutrons, 24 electrons
(b) 45 protons, 24 neutrons, 43 electrons
(c) 53 protons, 89 neutrons, 54 electrons
(d) 97 protons, 146 neutrons, 97 electrons
(a) (b) (c) (d)
Calculate the density of the nucleus in g/mL, assuming that it has the typical nuclear diameter of 1 10 –13 cm and is spherical in shape.
What are the two principal differences between nuclear reactions and ordinary chemical changes?
Nuclear reactions usually change one type of nucleus into another; chemical changes rearrange atoms. Nuclear reactions involve much larger energies than chemical reactions and have measureable mass changes.
The mass of the atom is 22.9898 amu.
(a) Calculate its binding energy per atom in millions of electron volts.
(b) Calculate its binding energy per nucleon.
Which of the following nuclei lie within the band of stability shown in [link] ?
(a) chlorine-37
(b) calcium-40
(c) 204 Bi
(d) 56 Fe
(e) 206 Pb
(f) 211 Pb
(g) 222 Rn
(h) carbon-14
(a), (b), (c), (d), and (e)
Which of the following nuclei lie within the band of stability shown in [link] ?
(a) argon-40
(b) oxygen-16
(c) 122 Ba
(d) 58 Ni
(e) 205 Tl
(f) 210 Tl
(g) 226 Ra
(h) magnesium-24

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?