| << Chapter < Page | Chapter >> Page > |
(a) from the ideal gas law
(b) from the van der Waals equation
(c) Explain the reason(s) for the difference.
(b) From the van der Waals equation:
This finally yields P = 32.4 atm.
(c) This is not very different from the value from the ideal gas law because the pressure is not very high and the temperature is not very low. The value is somewhat different because CO 2 molecules do have some volume and attractions between molecules, and the ideal gas law assumes they do not have volume or attractions.
(a) from the ideal gas law
(b) from the van der Waals equation
(c) Explain the reason(s) for the difference.
(a) 46.562 atm; (b) 46.594 atm; (c) The van der Waals equation takes into account the volume of the gas molecules themselves as well as intermolecular attractions.
Gas molecules possess a finite volume and experience forces of attraction for one another. Consequently, gas behavior is not necessarily described well by the ideal gas law. Under conditions of low pressure and high temperature, these factors are negligible, the ideal gas equation is an accurate description of gas behavior, and the gas is said to exhibit ideal behavior. However, at lower temperatures and higher pressures, corrections for molecular volume and molecular attractions are required to account for finite molecular size and attractive forces. The van der Waals equation is a modified version of the ideal gas law that can be used to account for the non-ideal behavior of gases under these conditions.
Graphs showing the behavior of several different gases follow. Which of these gases exhibit behavior significantly different from that expected for ideal gases?
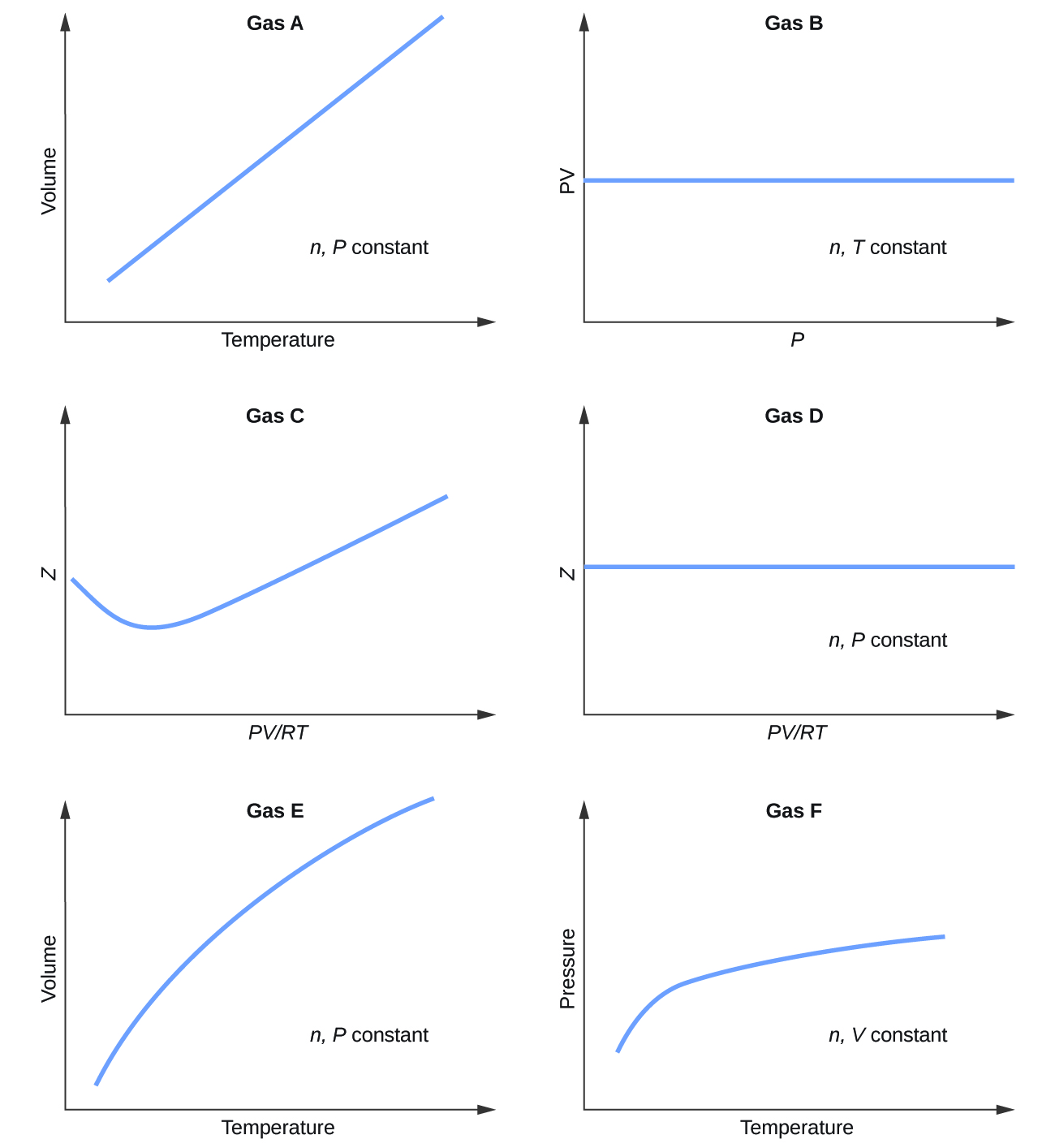
Gases C, E, and F
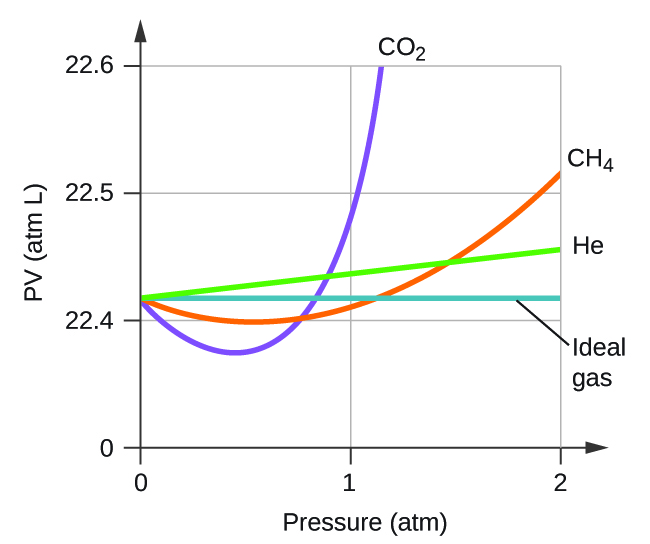
Explain why the plot of PV for CO 2 differs from that of an ideal gas.
Under which of the following sets of conditions does a real gas behave most like an ideal gas, and for which conditions is a real gas expected to deviate from ideal behavior? Explain.
(a) high pressure, small volume
(b) high temperature, low pressure
(c) low temperature, high pressure
The gas behavior most like an ideal gas will occur under the conditions in (b). Molecules have high speeds and move through greater distances between collision; they also have shorter contact times and interactions are less likely. Deviations occur with the conditions described in (a) and (c). Under conditions of (a), some gases may liquefy. Under conditions of (c), most gases will liquefy.
Describe the factors responsible for the deviation of the behavior of real gases from that of an
ideal gas.
For which of the following gases should the correction for the molecular volume be largest:
CO, CO 2 , H 2 , He, NH 3 , SF 6 ?
SF 6
A 0.245-L flask contains 0.467 mol CO 2 at 159 °C. Calculate the pressure:
(a) using the ideal gas law
(b) using the van der Waals equation
(c) Explain the reason for the difference.
(d) Identify which correction (that for P or V) is dominant and why.
Answer the following questions:
(a) If XX behaved as an ideal gas, what would its graph of Z vs. P look like?
(b) For most of this chapter, we performed calculations treating gases as ideal. Was this justified?
(c) What is the effect of the volume of gas molecules on Z? Under what conditions is this effect small? When is it large? Explain using an appropriate diagram.
(d) What is the effect of intermolecular attractions on the value of Z? Under what conditions is this effect small? When is it large? Explain using an appropriate diagram.
(e) In general, under what temperature conditions would you expect Z to have the largest deviations from the Z for an ideal gas?
(a) A straight horizontal line at 1.0; (b) When real gases are at low pressures and high temperatures they behave close enough to ideal gases that they are approximated as such, however, in some cases, we see that at a high pressure and temperature, the ideal gas approximation breaks down and is significantly different from the pressure calculated by the ideal gas equation (c) The greater the compressibility, the more the volume matters. At low pressures, the correction factor for intermolecular attractions is more significant, and the effect of the volume of the gas molecules on Z would be a small lowering compressibility. At higher pressures, the effect of the volume of the gas molecules themselves on Z would increase compressibility (see [link] ) (d) Once again, at low pressures, the effect of intermolecular attractions on Z would be more important than the correction factor for the volume of the gas molecules themselves, though perhaps still small. At higher pressures and low temperatures, the effect of intermolecular attractions would be larger. See [link] . (e) low temperatures

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?