| << Chapter < Page | Chapter >> Page > |
The half-life of a reaction is the time required to decrease the amount of a given reactant by one-half. The half-life of a zero-order reaction decreases as the initial concentration of the reactant in the reaction decreases. The half-life of a first-order reaction is independent of concentration, and the half-life of a second-order reaction decreases as the concentration increases.
Describe how graphical methods can be used to determine the order of a reaction and its rate constant from a series of data that includes the concentration of A at varying times.
Use the data provided to graphically determine the order and rate constant of the following reaction:
| Time (s) | 0 | 5.00 10 3 | 1.00 10 4 | 1.50 10 4 |
| [SO 2 Cl 2 ] ( M ) | 0.100 | 0.0896 | 0.0802 | 0.0719 |
| Time (s) | 2.50 10 4 | 3.00 10 4 | 4.00 10 4 | |
| [SO 2 Cl 2 ] ( M ) | 0.0577 | 0.0517 | 0.0415 |
Plotting a graph of ln[SO
2 Cl
2 ] versus
t reveals a linear trend; therefore we know this is a first-order reaction:
![A graph is shown with the label “Time ( s )” on the x-axis and “l n [ S O subscript 2 C l subscript 2 ] M” on the y-axis. The x-axis begins at 0 and extends to 4.00 times 10 superscript 4 with markings every 1.00 times 10 superscript 4. The y-axis shows markings extending from negative 3.5 to negative 2.5. A decreasing linear trend line is drawn through seven points at the approximate coordinates: (0, negative 2.3), (0.5 times 10 superscript 4, negative 2.4), (1.0 times 10 superscript 4, negative 2.5), (1.5 times 10 superscript 4, negative 2.6), (2.0 times 10 superscript 4, negative 2.9), (2.5 times 10 superscript 4, negative 3.0), and (3.0 times 10 superscript 4, negative 3.2).](/ocw/mirror/col11760/m51101/CNX_Chem_12_04_Exercise02_img.jpg)
k = −2.20
10
5 s
−1
Use the data provided in a graphical method to determine the order and rate constant of the following reaction:
| Time (s) | 9.0 | 13.0 | 18.0 | 22.0 | 25.0 |
| [P] ( M ) | 1.077 10 −3 | 1.068 10 −3 | 1.055 10 −3 | 1.046 10 −3 | 1.039 10 −3 |
Pure ozone decomposes slowly to oxygen,
Use the data provided in a graphical method and determine the order and rate constant of the reaction.
| Time (h) | 0 | 2.0 10 3 | 7.6 10 3 | 1.00 10 4 |
| [O 3 ] ( M ) | 1.00 10 −5 | 4.98 10 −6 | 2.07 10 −6 | 1.66 10 −6 |
| Time (h) | 1.23 10 4 | 1.43 10 4 | 1.70 10 4 | |
| [O 3 ] ( M ) | 1.39 10 −6 | 1.22 10 −6 | 1.05 10 −6 |
![A graph is shown with the label, “t ( h ,)” on the x-axis and, “1 divided by [ O subscript 3 ] M,” on the y-axis. The x-axis shows markings at 0, 2 times 10 superscript 3, 6 times 10 superscript 3, 10 time 10 superscript 3, 14 times 10 superscript 3, and 18 times 10 superscript 3. The y-axis shows markings beginning at 0, increasing by 1 up to and including 9. An increasing linear trend line is drawn through seven points at the coordinates: (0, 1.00), (2.0 times 10 superscript 3, 2.01), (7.6 times 10 superscript 3, 4.83), (1.00 times 10 superscript 4, 6.02), (1.23 times 10 superscript 4 , 6.02), (1.43 times 10 superscript 4, 8.20) and (1.70 times 10 superscript 4, 9.52). A horizontal line segment is drawn through the first point and a vertical line segment is similarly drawn through the last point to make a right triangle on the graph. The horizontal leg of the triangle is labeled “ capital delta t.” The vertical leg is labeled “capital delta 1 divided by [ O subscript 3 ].”](/ocw/mirror/col11760/m51101/CNX_Chem_12_04_Exercise04_img.jpg)
The plot is nicely linear, so the reaction is second order.
k = 50.1 L mol
−1 h
−1
From the given data, use a graphical method to determine the order and rate constant of the following reaction:
| Time (s) | 5.0 | 10.0 | 15.0 | 20.0 | 25.0 | 30.0 | 35.0 | 40.0 |
| [ X ] ( M ) | 0.0990 | 0.0497 | 0.0332 | 0.0249 | 0.0200 | 0.0166 | 0.0143 | 0.0125 |
What is the half-life for the first-order decay of phosphorus-32? The rate constant for the decay is 4.85 10 −2 day −1 .
14.3 d
What is the half-life for the first-order decay of carbon-14? The rate constant for the decay is 1.21 10 −4 year −1 .
What is the half-life for the decomposition of NOCl when the concentration of NOCl is 0.15 M ? The rate constant for this second-order reaction is 8.0 10 −8 L/mol/s.
8.3 10 7 s
What is the half-life for the decomposition of O 3 when the concentration of O 3 is 2.35 10 −6 M ? The rate constant for this second-order reaction is 50.4 L/mol/h.
The reaction of compound A to give compounds C and D was found to be second-order in A . The rate constant for the reaction was determined to be 2.42 L/mol/s. If the initial concentration is 0.500 mol/L, what is the value of t 1/2 ?
0.826 s
The half-life of a reaction of compound A to give compounds D and E is 8.50 min when the initial concentration of A is 0.150 mol/L. How long will it take for the concentration to drop to 0.0300 mol/L if the reaction is (a) first order with respect to A or (b) second order with respect to A ?
Some bacteria are resistant to the antibiotic penicillin because they produce penicillinase, an enzyme with a molecular weight of 3
10
4 g/mol that converts penicillin into inactive molecules. Although the kinetics of enzyme-catalyzed reactions can be complex, at low concentrations this reaction can be described by a rate equation that is first order in the catalyst (penicillinase) and that also involves the concentration of penicillin. From the following data: 1.0 L of a solution containing 0.15 µg (0.15
10
−6 g) of penicillinase, determine the order of the reaction with respect to penicillin and the value of the rate constant.
| [Penicillin] ( M ) | Rate (mol/L/min) |
|---|---|
| 2.0 10 −6 | 1.0 10 −10 |
| 3.0 10 −6 | 1.5 10 −10 |
| 4.0 10 −6 | 2.0 10 −10 |
The reaction is first order.
k = 1.0
10
7 mol
−1 min
−1
Both technetium-99 and thallium-201 are used to image heart muscle in patients with suspected heart problems. The half-lives are 6 h and 73 h, respectively. What percent of the radioactivity would remain for each of the isotopes after 2 days (48 h)?
There are two molecules with the formula C
3 H
6 . Propene,
is the monomer of the polymer polypropylene, which is used for indoor-outdoor carpets. Cyclopropane is used as an anesthetic:
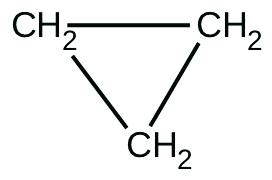
When heated to 499 °C, cyclopropane rearranges (isomerizes) and forms propene with a rate constant of
5.95
10
−4 s
−1 . What is the half-life of this reaction? What fraction of the cyclopropane remains after 0.75 h at 499.5 °C?
4.98; 20% remains
Fluorine-18 is a radioactive isotope that decays by positron emission to form oxygen-18 with a half-life of 109.7 min. (A positron is a particle with the mass of an electron and a single unit of positive charge; the equation is Physicians use 18 F to study the brain by injecting a quantity of fluoro-substituted glucose into the blood of a patient. The glucose accumulates in the regions where the brain is active and needs nourishment.
(a) What is the rate constant for the decomposition of fluorine-18?
(b) If a sample of glucose containing radioactive fluorine-18 is injected into the blood, what percent of the radioactivity will remain after 5.59 h?
(c) How long does it take for 99.99% of the 18 F to decay?
Suppose that the half-life of steroids taken by an athlete is 42 days. Assuming that the steroids biodegrade by a first-order process, how long would it take for of the initial dose to remain in the athlete’s body?
252 days
Recently, the skeleton of King Richard III was found under a parking lot in England. If tissue samples from the skeleton contain about 93.79% of the carbon-14 expected in living tissue, what year did King Richard III die? The half-life for carbon-14 is 5730 years.
Nitroglycerine is an extremely sensitive explosive. In a series of carefully controlled experiments, samples of the explosive were heated to 160 °C and their first-order decomposition studied. Determine the average rate constants for each experiment using the following data:
| Initial [C 3 H 5 N 3 O 9 ] ( M ) | 4.88 | 3.52 | 2.29 | 1.81 | 5.33 | 4.05 | 2.95 | 1.72 |
| t (s) | 300 | 300 | 300 | 300 | 180 | 180 | 180 | 180 |
| % Decomposed | 52.0 | 52.9 | 53.2 | 53.9 | 34.6 | 35.9 | 36.0 | 35.4 |
| [ A ] 0 ( M ) | k 10 3 (s −1 ) |
|---|---|
| 4.88 | 2.45 |
| 3.52 | 2.51 |
| 2.29 | 2.54 |
| 1.81 | 2.58 |
| 5.33 | 2.35 |
| 4.05 | 2.44 |
| 2.95 | 2.47 |
| 1.72 | 2.43 |
For the past 10 years, the unsaturated hydrocarbon 1,3-butadiene
has ranked 38th among the top 50 industrial chemicals. It is used primarily for the manufacture of synthetic rubber. An isomer exists also as cyclobutene:
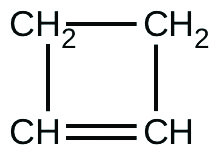
The isomerization of cyclobutene to butadiene is first-order and the rate constant has been measured as 2.0 10 −4 s −1 at 150 °C in a 0.53-L flask. Determine the partial pressure of cyclobutene and its concentration after 30.0 minutes if an isomerization reaction is carried out at 150 °C with an initial pressure of 55 torr.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?