| << Chapter < Page | Chapter >> Page > |
(a) What is its activity in Bq?
(b) What is its activity in Ci?
And to convert this to decays per second:
(a) Since 1 Bq = the activity in Becquerel (Bq) is:
(b) Since 1 Ci = the activity in curie (Ci) is:
(a) 3.56 10 11 Bq; (b) 0.962 Ci
The effects of radiation depend on the type, energy, and location of the radiation source, and the length of exposure. As shown in [link] , the average person is exposed to background radiation, including cosmic rays from the sun and radon from uranium in the ground (see the Chemistry in Everyday Life feature on Radon Exposure); radiation from medical exposure, including CAT scans, radioisotope tests, X-rays, and so on; and small amounts of radiation from other human activities, such as airplane flights (which are bombarded by increased numbers of cosmic rays in the upper atmosphere), radioactivity from consumer products, and a variety of radionuclides that enter our bodies when we breathe (for example, carbon-14) or through the food chain (for example, potassium-40, strontium-90, and iodine-131).
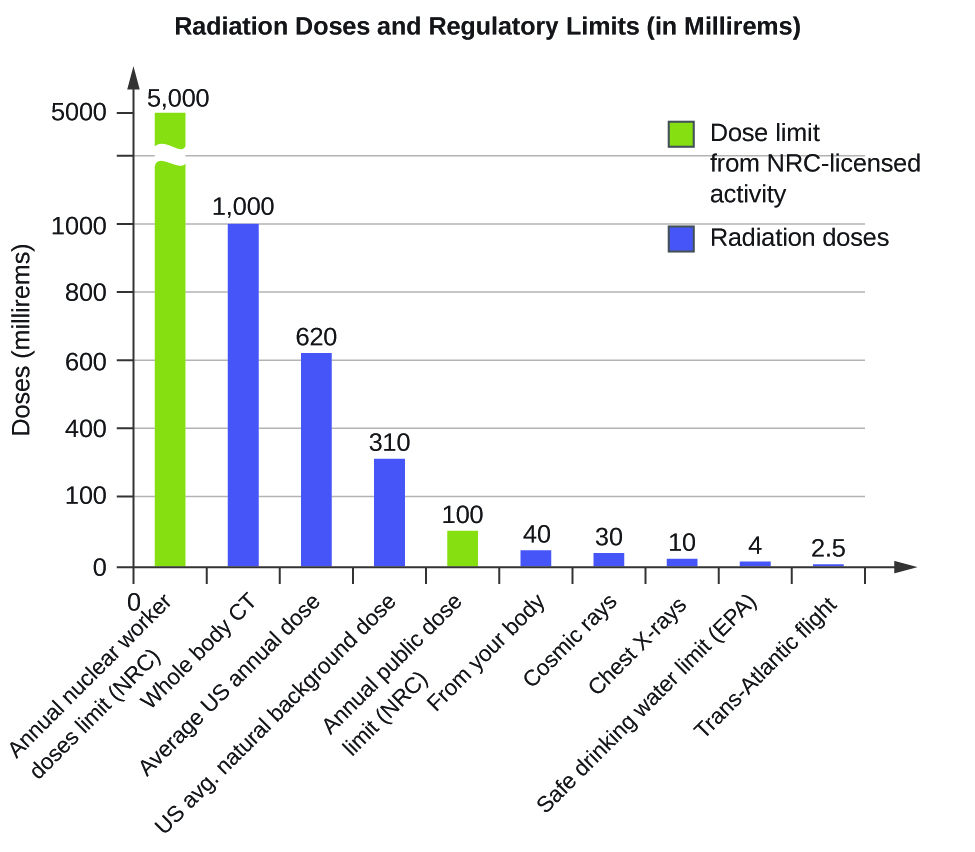
A short-term, sudden dose of a large amount of radiation can cause a wide range of health effects, from changes in blood chemistry to death. Short-term exposure to tens of rems of radiation will likely cause very noticeable symptoms or illness; a dose of about 500 rems is estimated to have a 50% probability of causing the death of the victim within 30 days of exposure. Exposure to radioactive emissions has a cumulative effect on the body during a person’s lifetime, which is another reason why it is important to avoid any unnecessary exposure to radiation. Health effects of short-term exposure to radiation are shown in [link] .
| Health Effects of Radiation Source: US Environmental Protection Agency | ||
|---|---|---|
| Exposure (rem) | Health Effect | Time to Onset (without treatment) |
| 5–10 | changes in blood chemistry | — |
| 50 | nausea | hours |
| 55 | fatigue | — |
| 70 | vomiting | — |
| 75 | hair loss | 2–3 weeks |
| 90 | diarrhea | — |
| 100 | hemorrhage | — |
| 400 | possible death | within 2 months |
| 1000 | destruction of intestinal lining | — |
| internal bleeding | — | |
| death | 1–2 weeks | |
| 2000 | damage to central nervous system | — |
| loss of consciousness; | minutes | |
| death | hours to days |
It is impossible to avoid some exposure to ionizing radiation. We are constantly exposed to background radiation from a variety of natural sources, including cosmic radiation, rocks, medical procedures, consumer products, and even our own atoms. We can minimize our exposure by blocking or shielding the radiation, moving farther from the source, and limiting the time of exposure.
We are constantly exposed to radiation from a variety of naturally occurring and human-produced sources. This radiation can affect living organisms. Ionizing radiation is the most harmful because it can ionize molecules or break chemical bonds, which damages the molecule and causes malfunctions in cell processes. It can also create reactive hydroxyl radicals that damage biological molecules and disrupt physiological processes. Radiation can cause somatic or genetic damage, and is most harmful to rapidly reproducing cells. Types of radiation differ in their ability to penetrate material and damage tissue, with alpha particles the least penetrating but potentially most damaging and gamma rays the most penetrating.
Various devices, including Geiger counters, scintillators, and dosimeters, are used to detect and measure radiation, and monitor radiation exposure. We use several units to measure radiation: becquerels or curies for rates of radioactive decay; gray or rads for energy absorbed; and rems or sieverts for biological effects of radiation. Exposure to radiation can cause a wide range of health effects, from minor to severe, and including death. We can minimize the effects of radiation by shielding with dense materials such as lead, moving away from the source, and limiting time of exposure.
If a hospital were storing radioisotopes, what is the minimum containment needed to protect against:
(a) cobalt-60 (a strong γ emitter used for irradiation)
(b) molybdenum-99 (a beta emitter used to produce technetium-99 for imaging)
Based on what is known about Radon-222’s primary decay method, why is inhalation so dangerous?
Alpha particles can be stopped by very thin shielding but have much stronger ionizing potential than beta particles, X-rays, and γ-rays. When inhaled, there is no protective skin covering the cells of the lungs, making it possible to damage the DNA in those cells and cause cancer.
Given specimens uranium-232 ( t 1/2 = 68.9 y) and uranium-233 ( t 1/2 = 159,200 y) of equal mass, which one would have greater activity and why?
A scientist is studying a 2.234 g sample of thorium-229 ( t 1/2 = 7340 y) in a laboratory.
(a) What is its activity in Bq?
(b) What is its activity in Ci?
(a) 7.64 10 9 Bq; (b) 2.06 10 −2 Ci
Given specimens neon-24 ( t 1/2 = 3.38 min) and bismuth-211 ( t 1/2 = 2.14 min) of equal mass, which one would have greater activity and why?

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?