| << Chapter < Page | Chapter >> Page > |
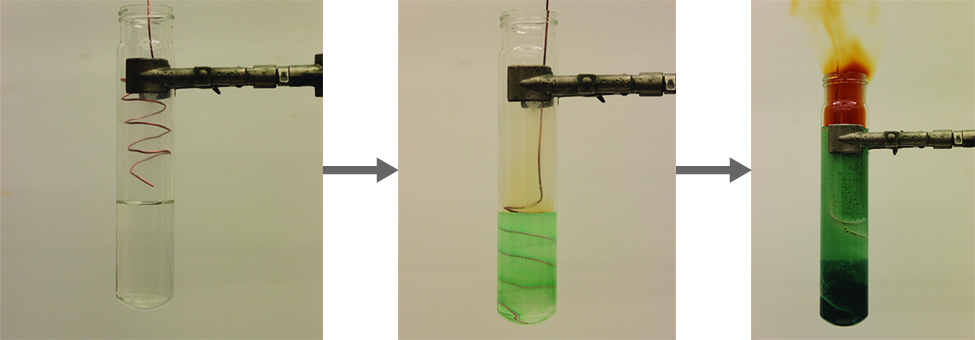
It is possible to prepare nitrogen dioxide in the laboratory by heating the nitrate of a heavy metal, or by the reduction of concentrated nitric acid with copper metal, as shown in [link] . Commercially, it is possible to prepare nitrogen dioxide by oxidizing nitric oxide with air.
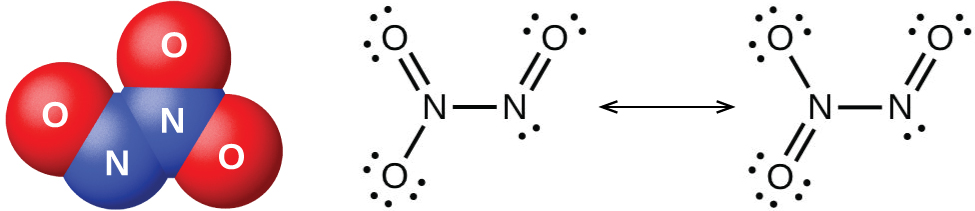
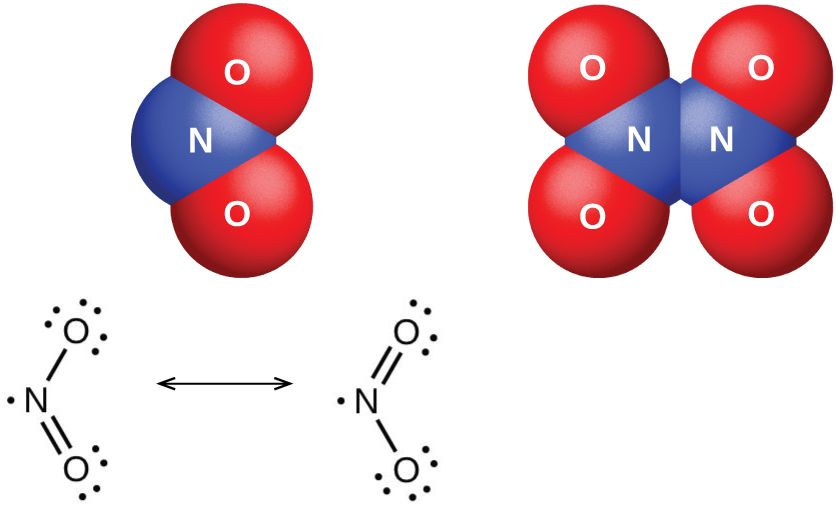
The nitrogen dioxide molecule (illustrated in [link] ) contains an unpaired electron, which is responsible for its color and paramagnetism. It is also responsible for the dimerization of NO 2 . At low pressures or at high temperatures, nitrogen dioxide has a deep brown color that is due to the presence of the NO 2 molecule. At low temperatures, the color almost entirely disappears as dinitrogen tetraoxide, N 2 O 4 , forms. At room temperature, an equilibrium exists:
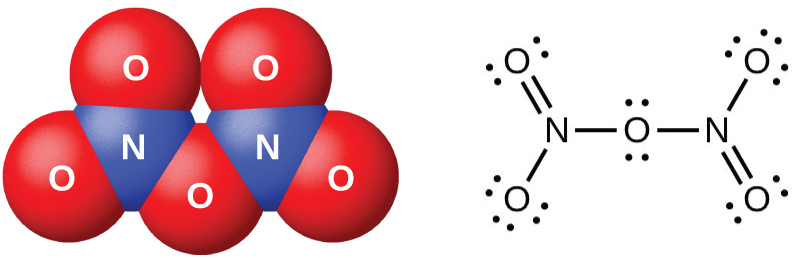
Dinitrogen pentaoxide, N 2 O 5 (illustrated in [link] ), is a white solid that is formed by the dehydration of nitric acid by phosphorus(V) oxide (tetraphosphorus decoxide):
It is unstable above room temperature, decomposing to N 2 O 4 and O 2 .
The oxides of nitrogen(III), nitrogen(IV), and nitrogen(V) react with water and form nitrogen-containing oxyacids. Nitrogen(III) oxide, N 2 O 3 , is the anhydride of nitrous acid; HNO 2 forms when N 2 O 3 reacts with water. There are no stable oxyacids containing nitrogen with an oxidation state of 4+; therefore, nitrogen(IV) oxide, NO 2 , disproportionates in one of two ways when it reacts with water. In cold water, a mixture of HNO 2 and HNO 3 forms. At higher temperatures, HNO 3 and NO will form. Nitrogen(V) oxide, N 2 O 5 , is the anhydride of nitric acid; HNO 3 is produced when N 2 O 5 reacts with water:
The nitrogen oxides exhibit extensive oxidation-reduction behavior. Nitrous oxide resembles oxygen in its behavior when heated with combustible substances. N 2 O is a strong oxidizing agent that decomposes when heated to form nitrogen and oxygen. Because one-third of the gas liberated is oxygen, nitrous oxide supports combustion better than air (one-fifth oxygen). A glowing splinter bursts into flame when thrust into a bottle of this gas. Nitric oxide acts both as an oxidizing agent and as a reducing agent. For example:
Nitrogen dioxide (or dinitrogen tetraoxide) is a good oxidizing agent. For example:
Nitrogen exhibits oxidation states ranging from 3− to 5+. Because of the stability of the N≡N triple bond, it requires a great deal of energy to make compounds from molecular nitrogen. Active metals such as the alkali metals and alkaline earth metals can reduce nitrogen to form metal nitrides. Nitrogen oxides and nitrogen hydrides are also important substances.
Write the Lewis structures for each of the following:
(a) NH 2−
(b) N 2 F 4
(c)
(d) NF 3
(e)
(a) NH
2− :
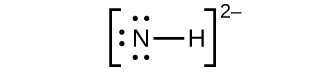 ;
;
(b) N
2 F
4 :
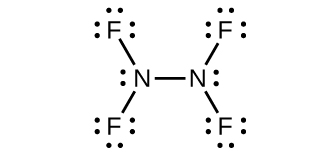 ;
;
(c)
 ;
;
(d) NF
3 :
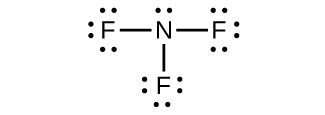 ;
;
(e)
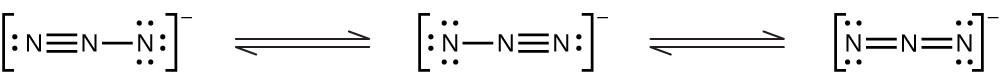
For each of the following, indicate the hybridization of the nitrogen atom (for the central nitrogen).
(a) N 2 F 4
(b)
(c) NF 3
(d)
Explain how ammonia can function both as a Brønsted base and as a Lewis base.
Ammonia acts as a Brønsted base because it readily accepts protons and as a Lewis base in that it has an electron pair to donate.
Brønsted base:
Lewis base:
Determine the oxidation state of nitrogen in each of the following. You may wish to review the chapter on chemical bonding for relevant examples.
(a) NCl 3
(b) ClNO
(c) N 2 O 5
(d) N 2 O 3
(e)
(f) N 2 O 4
(g) N 2 O
(h)
(i) HNO 2
(j) HNO 3
For each of the following, draw the Lewis structure, predict the ONO bond angle, and give the hybridization of the nitrogen. You may wish to review the chapters on chemical bonding and advanced theories of covalent bonding for relevant examples.
(a) NO 2
(b)
(c)
(a) NO
2 :
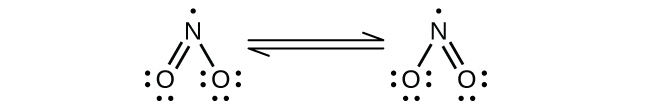
Nitrogen is
sp
2 hybridized. The molecule has a bent geometry with an ONO bond angle of approximately 120°.
(b)
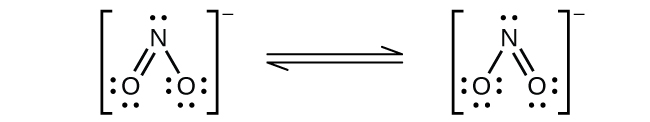
Nitrogen is
sp
2 hybridized. The molecule has a bent geometry with an ONO bond angle slightly less than 120°.
(c)
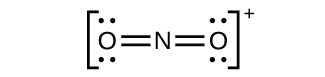
Nitrogen is
sp hybridized. The molecule has a linear geometry with an ONO bond angle of 180°.
How many grams of gaseous ammonia will the reaction of 3.0 g hydrogen gas and 3.0 g of nitrogen gas produce?
Although PF 5 and AsF 5 are stable, nitrogen does not form NF 5 molecules. Explain this difference among members of the same group.
Nitrogen cannot form a NF 5 molecule because it does not have d orbitals to bond with the additional two fluorine atoms.
The equivalence point for the titration of a 25.00-mL sample of CsOH solution with 0.1062 M HNO 3 is at 35.27 mL. What is the concentration of the CsOH solution?

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?