| << Chapter < Page | Chapter >> Page > |
Each cell produces 2 V, so six cells are connected in series to produce a 12-V car battery. Lead acid batteries are heavy and contain a caustic liquid electrolyte, but are often still the battery of choice because of their high current density. Since these batteries contain a significant amount of lead, they must always be disposed of properly.
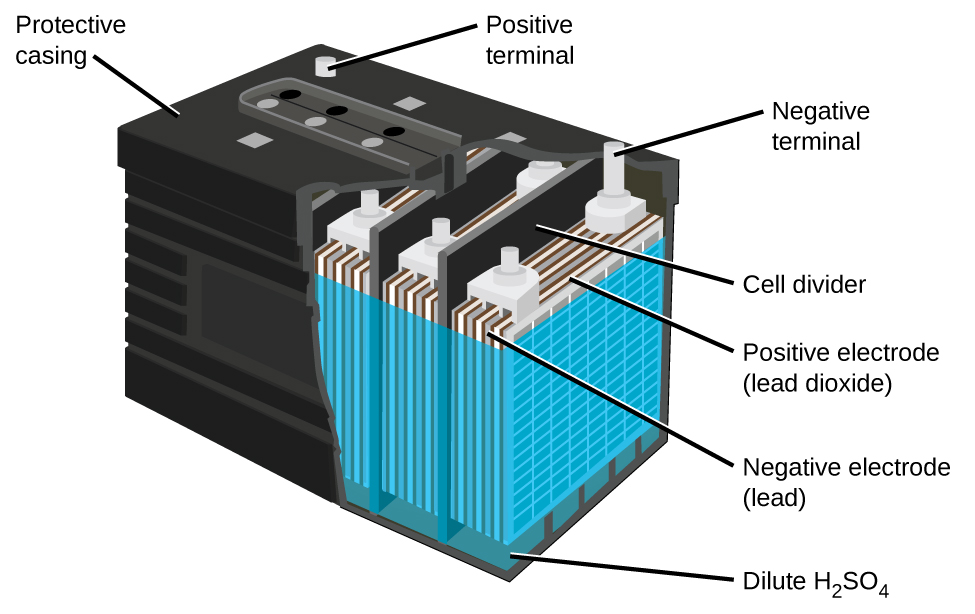
Visit this site for more information about lead acid batteries.
A fuel cell is a device that converts chemical energy into electrical energy. Fuel cells are similar to batteries but require a continuous source of fuel, often hydrogen. They will continue to produce electricity as long as fuel is available. Hydrogen fuel cells have been used to supply power for satellites, space capsules, automobiles, boats, and submarines ( [link] ).
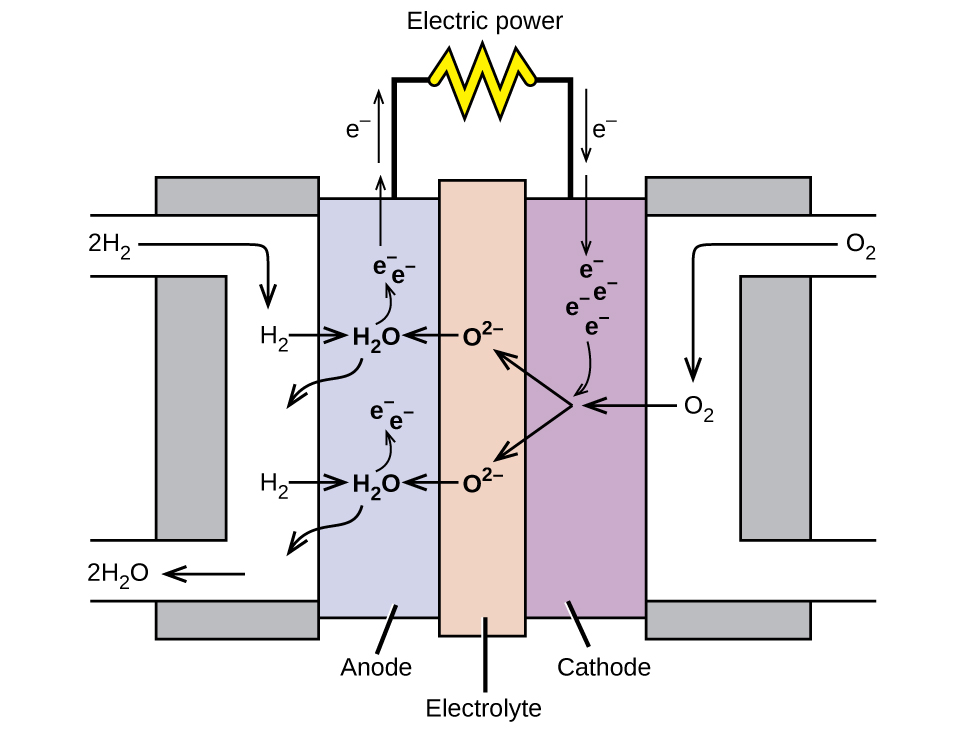
In a hydrogen fuel cell, the reactions are
The voltage is about 0.9 V. The efficiency of fuel cells is typically about 40% to 60%, which is higher than the typical internal combustion engine (25% to 35%) and, in the case of the hydrogen fuel cell, produces only water as exhaust. Currently, fuel cells are rather expensive and contain features that cause them to fail after a relatively short time.
Check out this link to learn more about fuel cells.
Batteries are galvanic cells, or a series of cells, that produce an electric current. When cells are combined into batteries, the potential of the battery is an integer multiple of the potential of a single cell. There are two basic types of batteries: primary and secondary. Primary batteries are “single use” and cannot be recharged. Dry cells and (most) alkaline batteries are examples of primary batteries. The second type is rechargeable and is called a secondary battery. Examples of secondary batteries include nickel-cadmium (NiCd), lead acid, and lithium ion batteries. Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. The hydrogen fuel cell uses hydrogen and oxygen from the air to produce water, and is generally more efficient than internal combustion engines.
What are the desirable qualities of an electric battery?
List some things that are typically considered when selecting a battery for a new application.
Considerations include: cost of the materials used in the battery, toxicity of the various components (what constitutes proper disposal), should it be a primary or secondary battery, energy requirements (the “size” of the battery/how long should it last), will a particular battery leak when the new device is used according to directions, and its mass (the total mass of the new device).
Consider a battery made from one half-cell that consists of a copper electrode in 1 M CuSO 4 solution and another half-cell that consists of a lead electrode in 1 M Pb(NO 3 ) 2 solution.
(a) What are the reactions at the anode, cathode, and the overall reaction?
(b) What is the standard cell potential for the battery?
(c) Most devices designed to use dry-cell batteries can operate between 1.0 and 1.5 V. Could this cell be used to make a battery that could replace a dry-cell battery? Why or why not.
(d) Suppose sulfuric acid is added to the half-cell with the lead electrode and some PbSO 4 ( s ) forms. Would the cell potential increase, decrease, or remain the same?
Consider a battery with the overall reaction:
(a) What is the reaction at the anode and cathode?
(b) A battery is “dead” when it has no cell potential. What is the value of Q when this battery is dead?
(c) If a particular dead battery was found to have [Cu 2+ ] = 0.11 M , what was the concentration of silver ion?
(a) (b) 3.5 10 15 ; (c) 5.6 10 −9 M
An inventor proposes using a SHE (standard hydrogen electrode) in a new battery for smartphones that also removes toxic carbon monoxide from the air:
Would this make a good battery for smartphones? Why or why not?
Why do batteries go dead, but fuel cells do not?
Batteries are self-contained and have a limited supply of reagents to expend before going dead. Alternatively, battery reaction byproducts accumulate and interfere with the reaction. Because a fuel cell is constantly resupplied with reactants and products are expelled, it can continue to function as long as reagents are supplied.
Explain what happens to battery voltage as a battery is used, in terms of the Nernst equation.
Using the information thus far in this chapter, explain why battery-powered electronics perform poorly in low temperatures.
E cell , as described in the Nernst equation, has a term that is directly proportional to temperature. At low temperatures, this term is decreased, resulting in a lower cell voltage provided by the battery to the device—the same effect as a battery running dead.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?