| << Chapter < Page | Chapter >> Page > |
Read the labels of several commercial products and identify monatomic ions of at least four transition elements contained in the products. Write the complete electron configurations of these cations.
Read the labels of several commercial products and identify monatomic ions of at least six main group elements contained in the products. Write the complete electron configurations of these cations and anions.
For example, Na + : 1 s 2 2 s 2 2 p 6 ; Ca 2+ : 1 s 2 2 s 2 2 p 6 ; Sn 2+ : 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 6 4 d 10 5 s 2 ; F – : 1 s 2 2 s 2 2 p 6 ; O 2– : 1 s 2 2 s 2 2 p 6 ; Cl – : 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 .
Using complete subshell notation (not abbreviations, 1 s 2 2 s 2 2 p 6 , and so forth), predict the electron configuration of each of the following atoms:
(a) C
(b) P
(c) V
(d) Sb
(e) Sm
Using complete subshell notation (1 s 2 2 s 2 2 p 6 , and so forth), predict the electron configuration of each of the following atoms:
(a) N
(b) Si
(c) Fe
(d) Te
(e) Tb
(a) 1 s 2 2 s 2 2 p 3 ; (b) 1 s 2 2 s 2 2 p 6 3 s 2 3 p 2 ; (c) 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 6 ; (d) 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 10 5 p 4 ; (e) 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 10 5 p 6 6 s 2 4 f 9
Is 1 s 2 2 s 2 2 p 6 the symbol for a macroscopic property or a microscopic property of an element? Explain your answer.
What additional information do we need to answer the question “Which ion has the electron configuration 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 ”?
The charge on the ion.
Draw the orbital diagram for the valence shell of each of the following atoms:
(a) C
(b) P
(c) V
(d) Sb
(e) Ru
Use an orbital diagram to describe the electron configuration of the valence shell of each of the following atoms:
(a) N
(b) Si
(c) Fe
(d) Te
(e) Mo
(a)
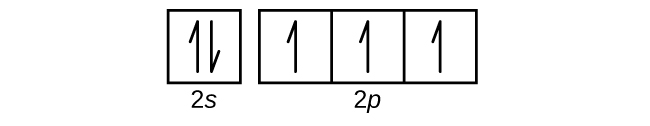
(b)
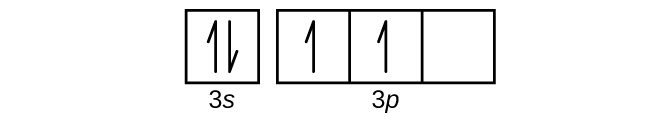
(c)
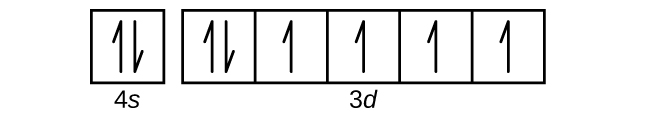
(d)
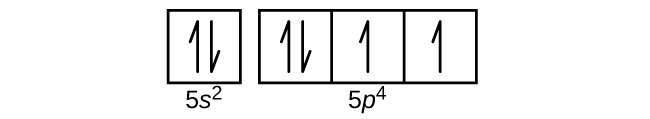
(e)
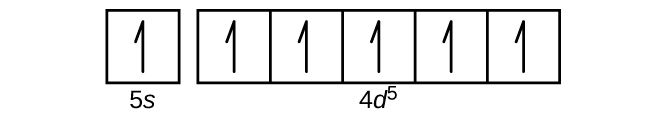
Using complete subshell notation (1 s 2 2 s 2 2 p 6 , and so forth), predict the electron configurations of the following ions.
(a) N 3–
(b) Ca 2+
(c) S –
(d) Cs 2+
(e) Cr 2+
(f) Gd 3+
Which atom has the electron configuration 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 2 ?
Zr
Which atom has the electron configuration 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 7 4 s 2 ?
Which ion with a +1 charge has the electron configuration 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 6 ? Which ion with a –2 charge has this configuration?
Rb + , Se 2−
Which of the following atoms contains only three valence electrons: Li, B, N, F, Ne?
Which of the following has two unpaired electrons?
(a) Mg
(b) Si
(c) S
(d) Both Mg and S
(e) Both Si and S.
Although both (b) and (c) are correct, (e) encompasses both and is the best answer.
Which atom would be expected to have a half-filled 6 p subshell?
Which atom would be expected to have a half-filled 4 s subshell?
K
In one area of Australia, the cattle did not thrive despite the presence of suitable forage. An investigation showed the cause to be the absence of sufficient cobalt in the soil. Cobalt forms cations in two oxidation states, Co 2+ and Co 3+ . Write the electron structure of the two cations.
Thallium was used as a poison in the Agatha Christie mystery story “The Pale Horse.” Thallium has two possible cationic forms, +1 and +3. The +1 compounds are the more stable. Write the electron structure of the +1 cation of thallium.
1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 6 4 d 10 5 s 2 5 p 6 6 s 2 4 f 14 5 d 10
Write the electron configurations for the following atoms or ions:
(a) B 3+
(b) O –
(c) Cl 3+
(d) Ca 2+
(e) Ti
Cobalt–60 and iodine–131 are radioactive isotopes commonly used in nuclear medicine. How many protons, neutrons, and electrons are in atoms of these isotopes? Write the complete electron configuration for each isotope.
Co has 27 protons, 27 electrons, and 33 neutrons: 1
s
2 2
s
2 2
p
6 3
s
2 3
p
6 4
s
2 3
d
7 .
I has 53 protons, 53 electrons, and 78 neutrons: 1
s
2 2
s
2 2
p
6 3
s
2 3
p
6 3
d
10 4
s
2 4
p
6 4
d
10 5
s
2 5
p
5 .
Write a set of quantum numbers for each of the electrons with an n of 3 in a Sc atom.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?