| << Chapter < Page | Chapter >> Page > |
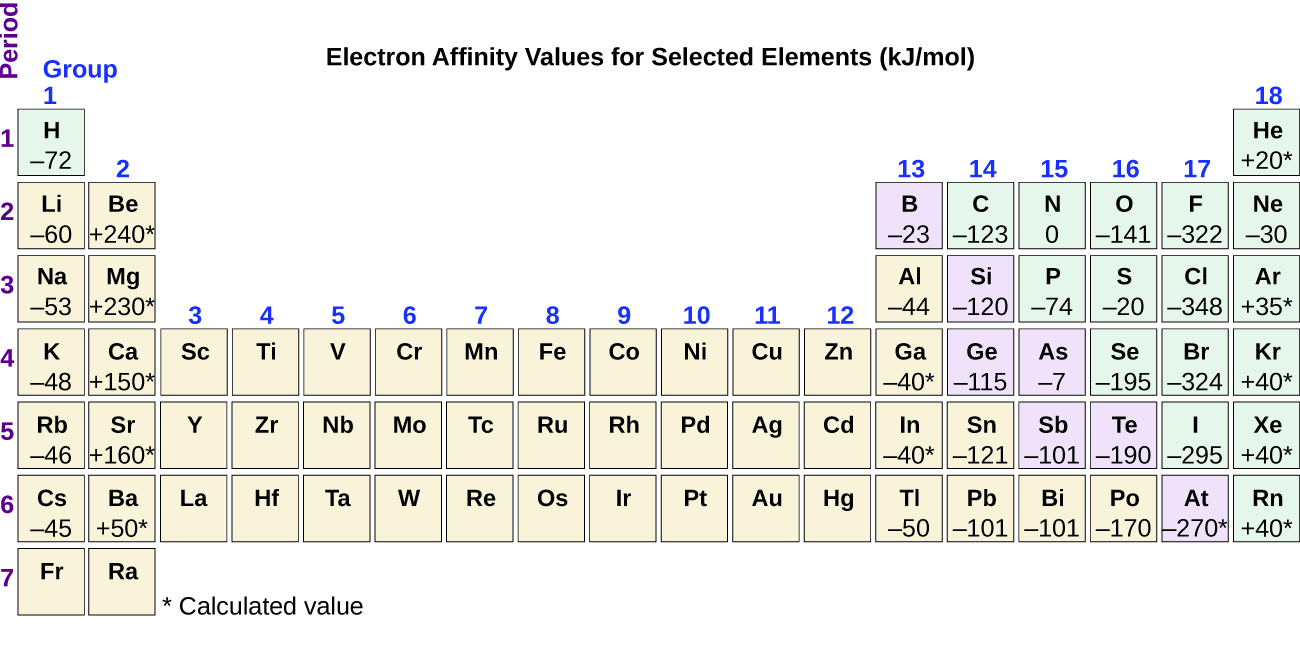
As we might predict, it becomes easier to add an electron across a series of atoms as the effective nuclear charge of the atoms increases. We find, as we go from left to right across a period, EAs tend to become more negative. The exceptions found among the elements of group 2 (2A), group 15 (5A), and group 18 (8A) can be understood based on the electronic structure of these groups. The noble gases, group 18 (8A), have a completely filled shell and the incoming electron must be added to a higher n level, which is more difficult to do. Group 2 (2A) has a filled ns subshell, and so the next electron added goes into the higher energy np , so, again, the observed EA value is not as the trend would predict. Finally, group 15 (5A) has a half-filled np subshell and the next electron must be paired with an existing np electron. In all of these cases, the initial relative stability of the electron configuration disrupts the trend in EA.
We also might expect the atom at the top of each group to have the largest EA; their first ionization potentials suggest that these atoms have the largest effective nuclear charges. However, as we move down a group, we see that the second element in the group most often has the greatest EA. The reduction of the EA of the first member can be attributed to the small size of the n = 2 shell and the resulting large electron–electron repulsions. For example, chlorine, with an EA value of –348 kJ/mol, has the highest value of any element in the periodic table. The EA of fluorine is –322 kJ/mol. When we add an electron to a fluorine atom to form a fluoride anion (F – ), we add an electron to the n = 2 shell. The electron is attracted to the nucleus, but there is also significant repulsion from the other electrons already present in this small valence shell. The chlorine atom has the same electron configuration in the valence shell, but because the entering electron is going into the n = 3 shell, it occupies a considerably larger region of space and the electron–electron repulsions are reduced. The entering electron does not experience as much repulsion and the chlorine atom accepts an additional electron more readily.
The properties discussed in this section (size of atoms and ions, effective nuclear charge, ionization energies, and electron affinities) are central to understanding chemical reactivity. For example, because fluorine has an energetically favorable EA and a large energy barrier to ionization (IE), it is much easier to form fluorine anions than cations. Metallic properties including conductivity and malleability (the ability to be formed into sheets) depend on having electrons that can be removed easily. Thus, metallic character increases as we move down a group and decreases across a period in the same trend observed for atomic size because it is easier to remove an electron that is farther away from the nucleus.
Electron configurations allow us to understand many periodic trends. Covalent radius increases as we move down a group because the n level (orbital size) increases. Covalent radius mostly decreases as we move left to right across a period because the effective nuclear charge experienced by the electrons increases, and the electrons are pulled in tighter to the nucleus. Anionic radii are larger than the parent atom, while cationic radii are smaller, because the number of valence electrons has changed while the nuclear charge has remained constant. Ionization energy (the energy associated with forming a cation) decreases down a group and mostly increases across a period because it is easier to remove an electron from a larger, higher energy orbital. Electron affinity (the energy associated with forming an anion) is more favorable (exothermic) when electrons are placed into lower energy orbitals, closer to the nucleus. Therefore, electron affinity becomes increasingly negative as we move left to right across the periodic table and decreases as we move down a group. For both IE and electron affinity data, there are exceptions to the trends when dealing with completely filled or half-filled subshells.
Based on their positions in the periodic table, predict which has the smallest atomic radius: Mg, Sr, Si, Cl, I.
Cl
Based on their positions in the periodic table, predict which has the largest atomic radius: Li, Rb, N, F, I.
Based on their positions in the periodic table, predict which has the largest first ionization energy: Mg, Ba, B, O, Te.
O
Based on their positions in the periodic table, predict which has the smallest first ionization energy: Li, Cs, N, F, I.
Based on their positions in the periodic table, rank the following atoms in order of increasing first ionization energy: F, Li, N, Rb
Rb<Li<N<F
Based on their positions in the periodic table, rank the following atoms or compounds in order of increasing first ionization energy: Mg, O, S, Si
Atoms of which group in the periodic table have a valence shell electron configuration of ns 2 np 3 ?
15 (5A)
Atoms of which group in the periodic table have a valence shell electron configuration of ns 2 ?
Based on their positions in the periodic table, list the following atoms in order of increasing radius: Mg, Ca, Rb, Cs.
Mg<Ca<Rb<Cs
Based on their positions in the periodic table, list the following atoms in order of increasing radius: Sr, Ca, Si, Cl.
Based on their positions in the periodic table, list the following ions in order of increasing radius: K + , Ca 2+ , Al 3+ , Si 4+ .
Si 4+ <Al 3+ <Ca 2+ <K +
List the following ions in order of increasing radius: Li + , Mg 2+ , Br – , Te 2– .
Which atom and/or ion is (are) isoelectronic with Br + : Se 2+ , Se, As – , Kr, Ga 3+ , Cl – ?
Se, As −
Which of the following atoms and ions is (are) isoelectronic with S 2+ : Si 4+ , Cl 3+ , Ar, As 3+ , Si, Al 3+ ?
Compare both the numbers of protons and electrons present in each to rank the following ions in order of increasing radius: As 3– , Br – , K + , Mg 2+ .
Mg 2+ <K + <Br – <As 3–
Of the five elements Al, Cl, I, Na, Rb, which has the most exothermic reaction? (E represents an atom.) What name is given to the energy for the reaction? Hint: note the process depicted does
not correspond to electron affinity
Of the five elements Sn, Si, Sb, O, Te, which has the most endothermic reaction? (E represents an atom.) What name is given to the energy for the reaction?
O, IE 1
The ionic radii of the ions S 2– , Cl – , and K + are 184, 181, 138 pm respectively. Explain why these ions have different sizes even though they contain the same number of electrons.
Which main group atom would be expected to have the lowest second ionization energy?
Ra
Explain why Al is a member of group 13 rather than group 3?

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?