| << Chapter < Page | Chapter >> Page > |
There are several different equations that better approximate gas behavior than does the ideal gas law. The first, and simplest, of these was developed by the Dutch scientist Johannes van der Waals in 1879. The van der Waals equation improves upon the ideal gas law by adding two terms: one to account for the volume of the gas molecules and another for the attractive forces between them.
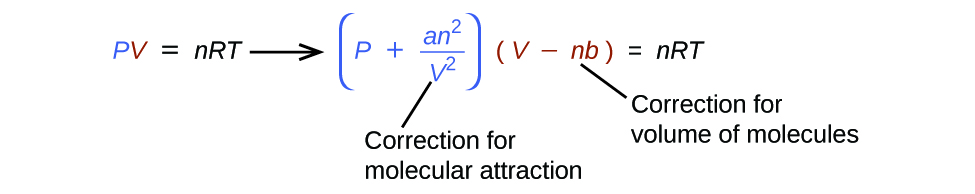
The constant a corresponds to the strength of the attraction between molecules of a particular gas, and the constant b corresponds to the size of the molecules of a particular gas. The “correction” to the pressure term in the ideal gas law is and the “correction” to the volume is nb . Note that when V is relatively large and n is relatively small, both of these correction terms become negligible, and the van der Waals equation reduces to the ideal gas law, PV = nRT . Such a condition corresponds to a gas in which a relatively low number of molecules is occupying a relatively large volume, that is, a gas at a relatively low pressure. Experimental values for the van der Waals constants of some common gases are given in [link] .
| Values of van der Waals Constants for Some Common Gases | ||
|---|---|---|
| Gas | a (L 2 atm/mol 2 ) | b (L/mol) |
| N 2 | 1.39 | 0.0391 |
| O 2 | 1.36 | 0.0318 |
| CO 2 | 3.59 | 0.0427 |
| H 2 O | 5.46 | 0.0305 |
| He | 0.0342 | 0.0237 |
| CCl 4 | 20.4 | 0.1383 |
At low pressures, the correction for intermolecular attraction, a , is more important than the one for molecular volume, b . At high pressures and small volumes, the correction for the volume of the molecules becomes important because the molecules themselves are incompressible and constitute an appreciable fraction of the total volume. At some intermediate pressure, the two corrections have opposing influences and the gas appears to follow the relationship given by PV = nRT over a small range of pressures. This behavior is reflected by the “dips” in several of the compressibility curves shown in [link] . The attractive force between molecules initially makes the gas more compressible than an ideal gas, as pressure is raised (Z decreases with increasing P ). At very high pressures, the gas becomes less compressible (Z increases with P ), as the gas molecules begin to occupy an increasingly significant fraction of the total gas volume.
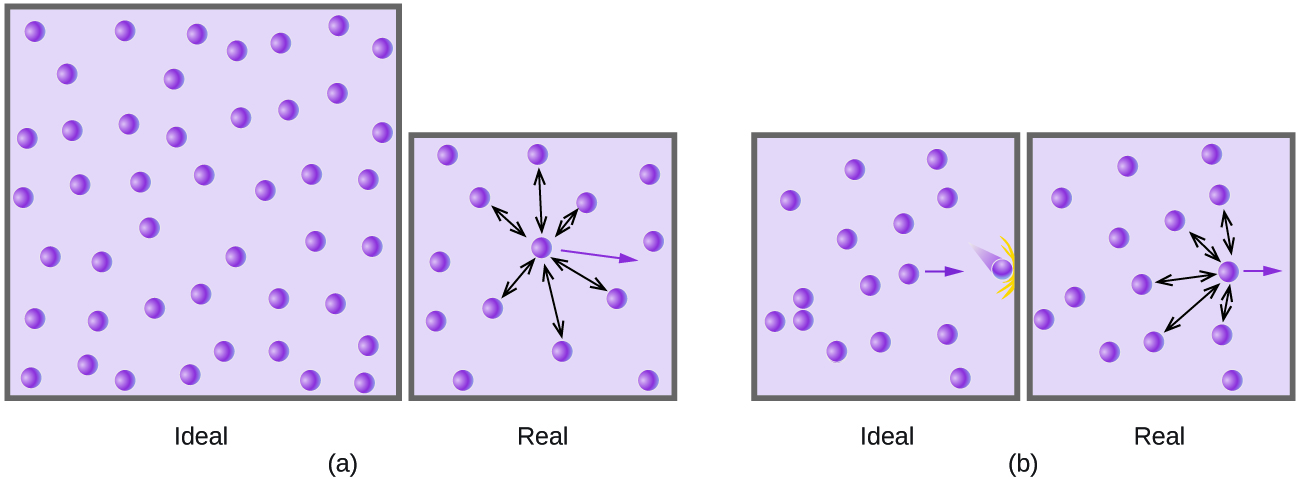
Strictly speaking, the ideal gas equation functions well when intermolecular attractions between gas molecules are negligible and the gas molecules themselves do not occupy an appreciable part of the whole volume. These criteria are satisfied under conditions of low pressure and high temperature . Under such conditions, the gas is said to behave ideally, and deviations from the gas laws are small enough that they may be disregarded—this is, however, very often not the case.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?