| << Chapter < Page | Chapter >> Page > |
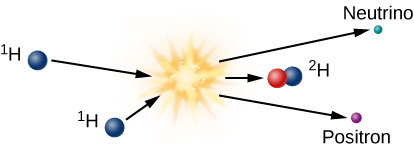
Since it is antimatter, this positron will instantly collide with a nearby electron, and both will be annihilated, producing electromagnetic energy in the form of gamma-ray photons. This gamma ray, which has been created in the center of the Sun, finds itself in a world crammed full of fast-moving nuclei and electrons. The gamma ray collides with particles of matter and transfers its energy to one of them. The particle later emits another gamma-ray photon, but often the emitted photon has a bit less energy than the one that was absorbed.
Such interactions happen to gamma rays again and again and again as they make their way slowly toward the outer layers of the Sun, until their energy becomes so reduced that they are no longer gamma rays but X-rays (recall what you learned in The Electromagnetic Spectrum ). Later, as the photons lose still more energy through collisions in the crowded center of the Sun, they become ultraviolet photons.
By the time they reach the Sun’s surface, most of the photons have given up enough energy to be ordinary light—and they are the sunlight we see coming from our star. (To be precise, each gamma-ray photon is ultimately converted into many separate lower-energy photons of sunlight.) So, the sunlight given off by the Sun today had its origin as a gamma ray produced by nuclear reactions deep in the Sun’s core. The length of time that photons require to reach the surface depends on how far a photon on average travels between collisions, and the travel time depends on what model of the complicated solar interior we accept. Estimates are somewhat uncertain but indicate that the emission of energy from the surface of the Sun can lag its production in the interior by 100,000 years to as much as 1,000,000 years.
In addition to the positron, the fusion of two hydrogen atoms to form deuterium results in the emission of a neutrino . Because neutrinos interact so little with ordinary matter, those produced by fusion reactions near the center of the Sun travel directly to the Sun’s surface and then out into space, in all directions. Neutrinos move at nearly the speed of light, and they escape the Sun about two seconds after they are created.

Notification Switch
Would you like to follow the 'Astronomy' conversation and receive update notifications?