| << Chapter < Page | Chapter >> Page > |
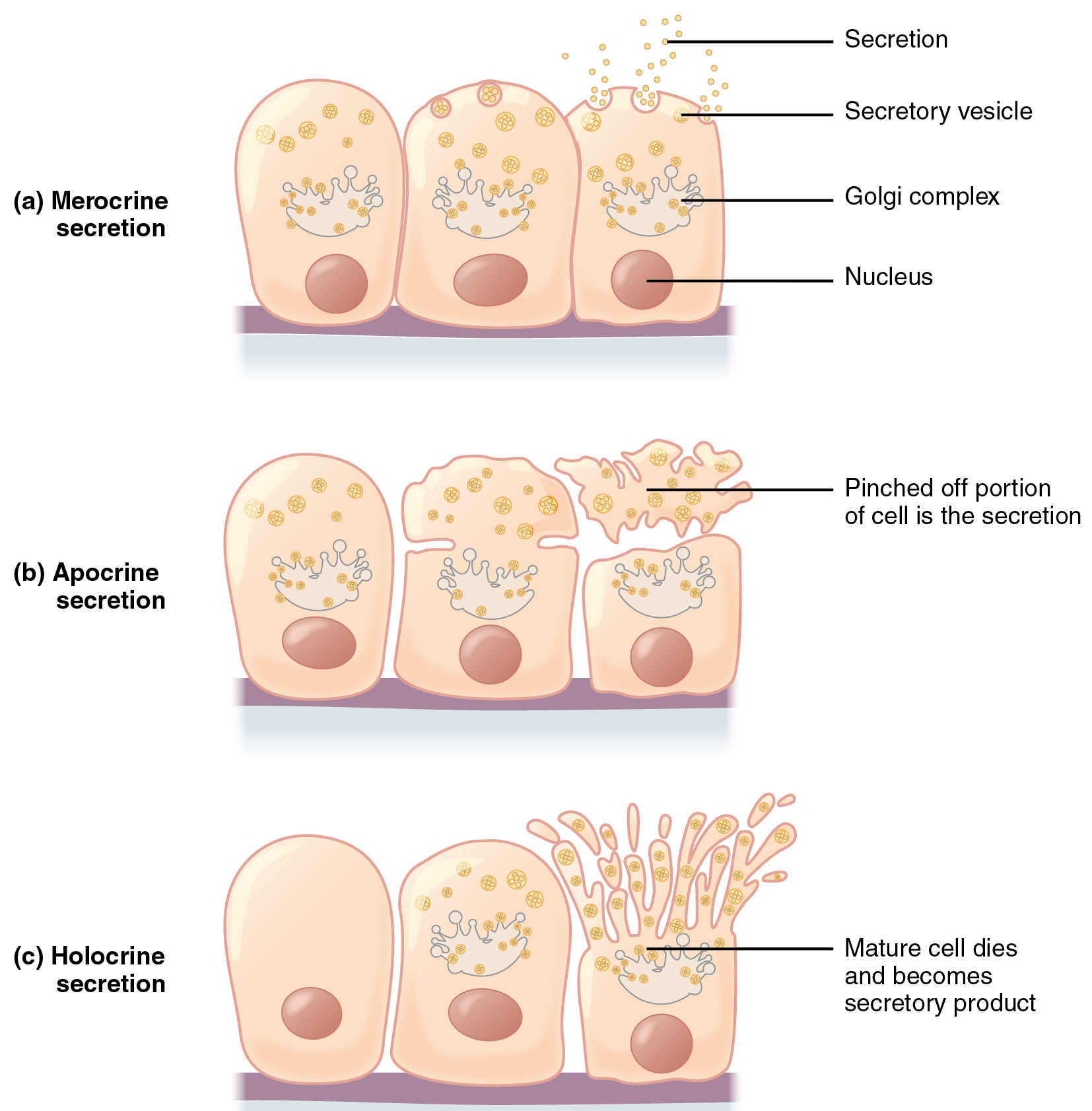
Apocrine secretion accumulates near the apical portion of the cell. That portion of the cell and its secretory contents pinch off from the cell and are released. The sweat glands of the armpit are classified as apocrine glands. Both merocrine and apocrine glands continue to produce and secrete their contents with little damage caused to the cell because the nucleus and golgi regions remain intact after secretion.
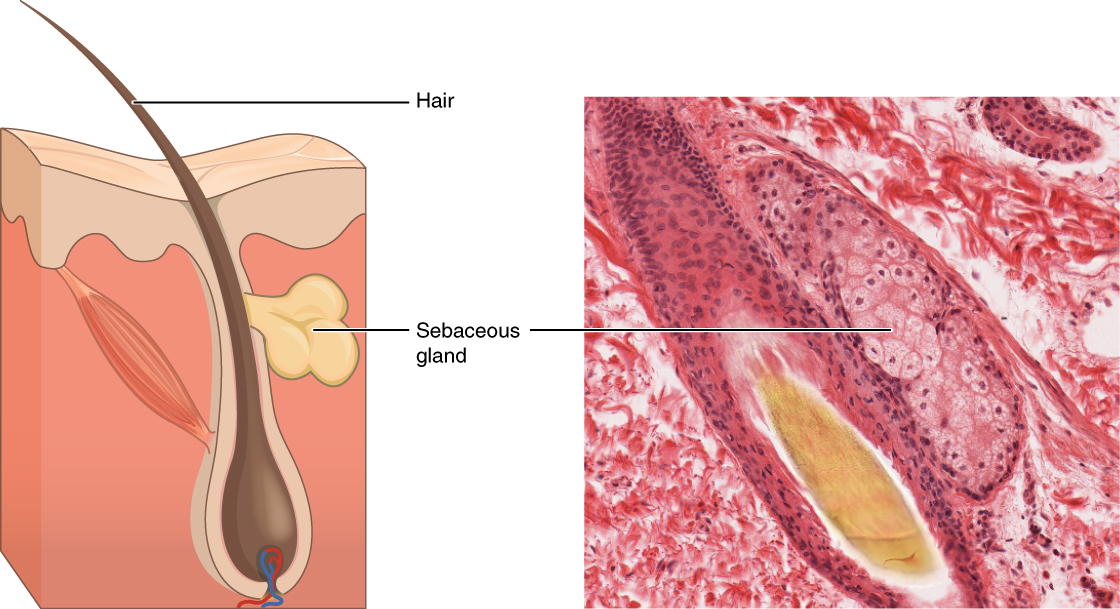
In contrast, the process of holocrine secretion involves the rupture and destruction of the entire gland cell. The cell accumulates its secretory products and releases them only when it bursts. New gland cells differentiate from cells in the surrounding tissue to replace those lost by secretion. The sebaceous glands that produce the oils on the skin and hair are holocrine glands/cells ( [link] ).
Glands are also named after the products they produce. The serous gland produces watery, blood-plasma-like secretions rich in enzymes such as alpha amylase, whereas the mucous gland releases watery to viscous products rich in the glycoprotein mucin. Both serous and mucous glands are common in the salivary glands of the mouth. Mixed exocrine glands contain both serous and mucous glands and release both types of secretions.
In epithelial tissue, cells are closely packed with little or no extracellular matrix except for the basal lamina that separates the epithelium from underlying tissue. The main functions of epithelia are protection from the environment, coverage, secretion and excretion, absorption, and filtration. Cells are bound together by tight junctions that form an impermeable barrier. They can also be connected by gap junctions, which allow free exchange of soluble molecules between cells, and anchoring junctions, which attach cell to cell or cell to matrix. The different types of epithelial tissues are characterized by their cellular shapes and arrangements: squamous, cuboidal, or columnar epithelia. Single cell layers form simple epithelia, whereas stacked cells form stratified epithelia. Very few capillaries penetrate these tissues.
Glands are secretory tissues and organs that are derived from epithelial tissues. Exocrine glands release their products through ducts. Endocrine glands secrete hormones directly into the interstitial fluid and blood stream. Glands are classified both according to the type of secretion and by their structure. Merocrine glands secrete products as they are synthesized. Apocrine glands release secretions by pinching off the apical portion of the cell, whereas holocrine gland cells store their secretions until they rupture and release their contents. In this case, the cell becomes part of the secretion.
Watch this video to find out more about the anatomy of epithelial tissues. Where in the body would one find non-keratinizing stratified squamous epithelium?
The inside of the mouth, esophagus, vaginal canal, and anus.

Notification Switch
Would you like to follow the 'Anatomy & Physiology' conversation and receive update notifications?