| << Chapter < Page | Chapter >> Page > |
1/P = k × V
This also means that the pressure must simply be a constant, k, multiplied by 1/V.
Of course, this result applies only to the single sample we have taken so far – we have trapped an arbitrary amount of air in our cylinder. What would happen if we trapped a different quantity of gas or if we trapped a different gas than the mixture of gases in air?
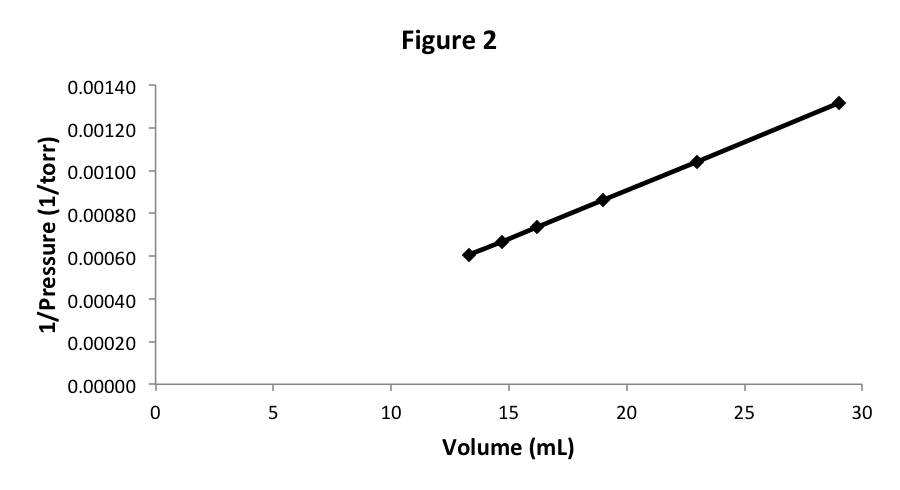
Let’s start with 29 mL of N 2 at pressure 760 torr, which are the same starting conditions for our previous sample of air. As we vary the volume and measure the pressure changes, we discover that the data is, to a very high accuracy, the same. We can repeat this experiment with O 2 , Ar, Ne, H 2 , or CH 4 , and we find in each case that if we start with the same volume and pressure, the pressure-volume data does not vary. In other words, the experimental graph in Figure 2 does not depend on what gas we are studying. That is fairly surprising, since we know that the particles (atoms or molecules) of these gases have very different properties.
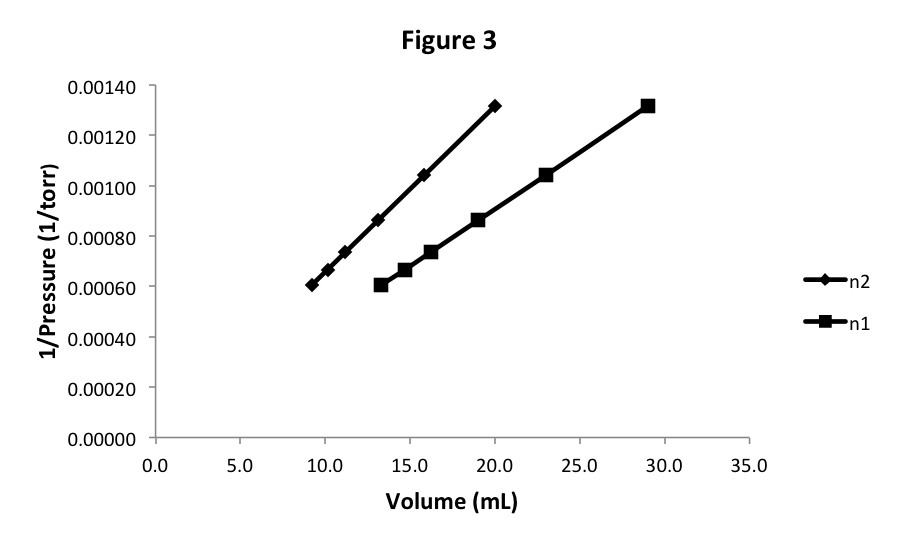
Let’s start with 20 mL of N 2 at pressure 760 torr. We could easily determine the mass of N 2 in the cylinder and show that there is less gas than in the previous experiment. We could also apply our knowledge of Avogadro’s Law and conclude that the volume of a gas is proportional to the number of particles or moles of the gas at the same pressure (and temperature). In either case, we know that we have fewer gas particles in the new sample. For this new sample, we observe that the pressure is lower at each volume, but the graph looks similar as seen in Figure 3.
Notice that we get another straight line that appears to pass through (0,0), so again the equation of this graph is 1/P = k × V. However, the slope of the line, or the value of k,is different. Since we changed the number of moles of gas between the two experiments, this means that the slope k is a function of the number of moles: k(n). We will later determine what this function of n is.
For now, we can conclude that 1/P is proportional to V for each value of n. Written differently:
P × V = k(n) at fixed temperature
In the next section, we will see that k also depends on temperature. Thus, we conclude that the product of pressure and volume is a constant for a given amount of gas at a fixed temperature . This observation is referred to as Boyle’s Law, dating to 1662.
We now want to measure what happens to the pressure and volume of a sample of gas when the temperature is allowed to vary. An interesting first problem that might not have been expected is the question of how to measure temperature. In fact, for most purposes, we think of temperature only in the rather non-quantitative manner of "how hot or cold" something is. However, we measure temperature in a variety of ways that do not seem to be “hot or cold,” such as by examining the length of mercury in a tube or by measuring the electrical potential across a thermocouple in an electronic thermometer. It is an important but complicated question of just what we are measuring when we measure the temperature.

Notification Switch
Would you like to follow the 'Concept development studies in chemistry 2013' conversation and receive update notifications?