| << Chapter < Page | Chapter >> Page > |
By the end of this section, you will be able to:
We can use Bohr’s model of the atom to understand how spectral lines are formed. The concept of energy levels for the electron orbits in an atom leads naturally to an explanation of why atoms absorb or emit only specific energies or wavelengths of light.
Let’s look at the hydrogen atom from the perspective of the Bohr model . Suppose a beam of white light (which consists of photons of all visible wavelengths) shines through a gas of atomic hydrogen. A photon of wavelength 656 nanometers has just the right energy to raise an electron in a hydrogen atom from the second to the third orbit. Thus, as all the photons of different energies (or wavelengths or colors) stream by the hydrogen atoms, photons with this particular wavelength can be absorbed by those atoms whose electrons are orbiting on the second level. When they are absorbed, the electrons on the second level will move to the third level, and a number of the photons of this wavelength and energy will be missing from the general stream of white light.
Other photons will have the right energies to raise electrons from the second to the fourth orbit, or from the first to the fifth orbit, and so on. Only photons with these exact energies can be absorbed. All of the other photons will stream past the atoms untouched. Thus, hydrogen atoms absorb light at only certain wavelengths and produce dark lines at those wavelengths in the spectrum we see.
Suppose we have a container of hydrogen gas through which a whole series of photons is passing, allowing many electrons to move up to higher levels. When we turn off the light source, these electrons “fall” back down from larger to smaller orbits and emit photons of light—but, again, only light of those energies or wavelengths that correspond to the energy difference between permissible orbits. The orbital changes of hydrogen electrons that give rise to some spectral lines are shown in [link] .
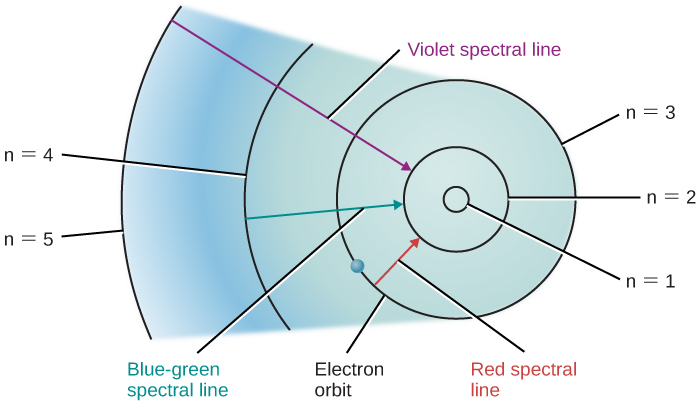
Similar pictures can be drawn for atoms other than hydrogen. However, because these other atoms ordinarily have more than one electron each, the orbits of their electrons are much more complicated, and the spectra are more complex as well. For our purposes, the key conclusion is this: each type of atom has its own unique pattern of electron orbits, and no two sets of orbits are exactly alike . This means that each type of atom shows its own unique set of spectral lines, produced by electrons moving between its unique set of orbits.

Notification Switch
Would you like to follow the 'Astronomy' conversation and receive update notifications?