| << Chapter < Page | Chapter >> Page > |
Linear dicrosim (LD) is also a very closely related technique to CD in which the difference between absorbance of perpendicularly and parallelly polarized light is measured. In this technique the plane of polaliration of light does not rotate. LD is used to determine the orientation of absorbing parts in space.
Just like any other instrument CD has its strengths and limits. The comparison between CD and NMR shown in [link] gives a good sense of capabilities of CD.
| CD | NMR |
| Molecules of any size can be studied | There is size limitation |
| The experiments are quick to perform; single wavelength measurements require milliseconds. | This is not the case all of the time. |
| Unique sensitivity to asymmetry in sample’s structure. | Special conditions are required to differentiate between enantiomers. |
| Can work with very small concentrations, by lengthening the cuvette width until discernable absorption is achieved. | There is a limit to sensitivity of instrument. |
| Timescale is much shorter (UV) thus allowing to study dynamic systems and kinetics. | Timescale is long, use of radio waves gives average of all dynamic systems. |
| Only qualitative analysis of data is possible. | Quantitative data analysis can be performed to estimate chemical composition. |
| Does not provide atomic level structure analysis | Very powerful for atomic level analysis, providing essential information about chemical bonds in system. |
| The observed spectrum is not enough for claiming one and only possible structure | The NMR spectrum is key information for assigning a unique structure. |
One effective way to demonstrate capabilities of CD spectroscopy is to cover the protein secondary structure study case, since CD spectroscopy is well-established technique for elucidation of secondary structure of proteins as well as any other macromolecules. By using CD one can estimate the degree of conformational order (what percent of the sample proteins is in α-helix and/or β-sheet conformation), see [link] .
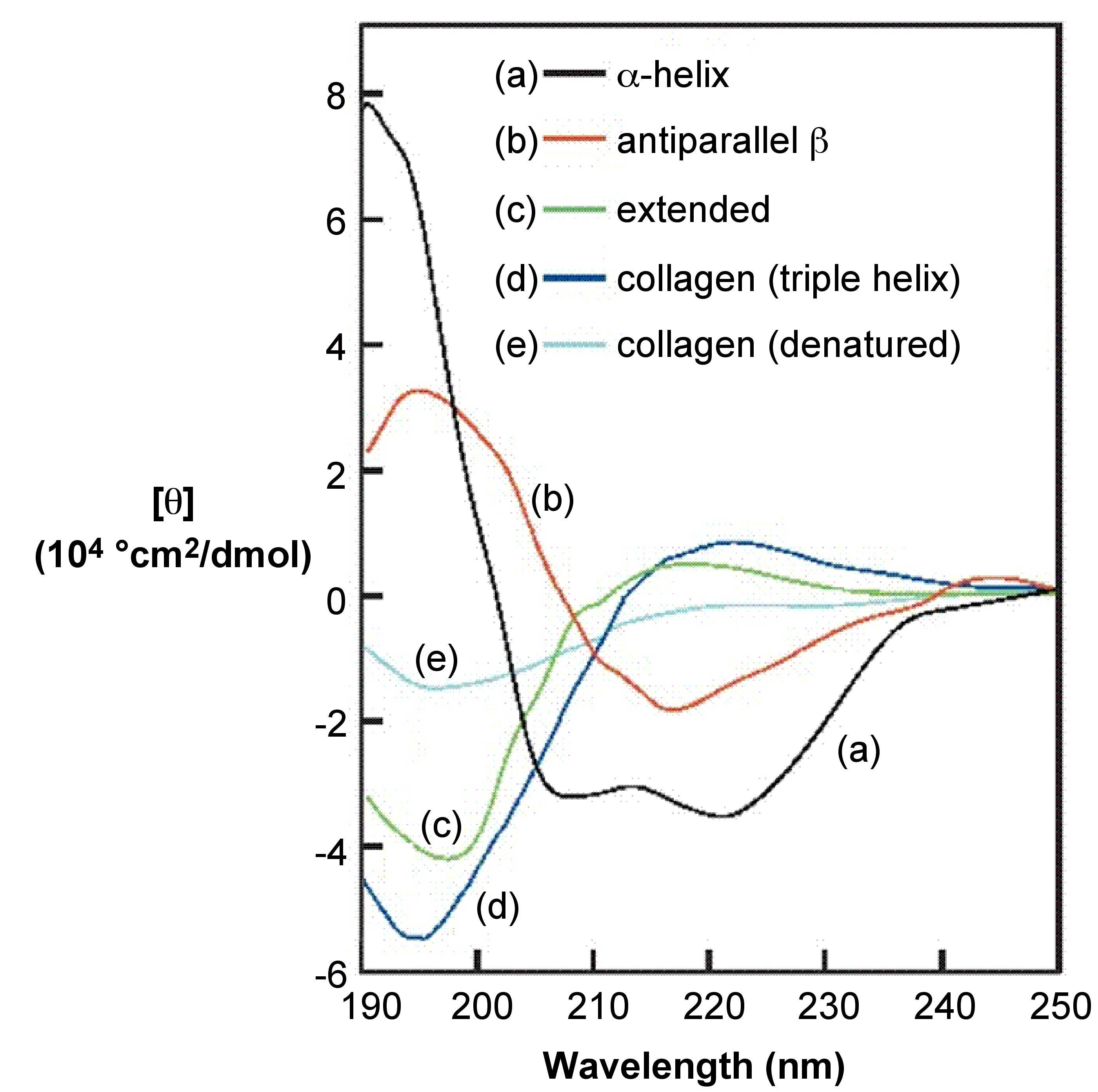
Key points for visual estimation of secondary structure by looking at a CD spectrum:
Since the CD spectra of proteins uniquely represent their conformation, CD can be used to monitor structural changes (due to complex formation, folding/unfolding, denaturation because of rise in temperature, denaturants, change in amino acid sequence/mutation, etc. ) in dynamic systems and to study kinetics of protein. In other words CD can be used to perform stability investigations and interaction modeling.
[link] shows a typical CD instrument.
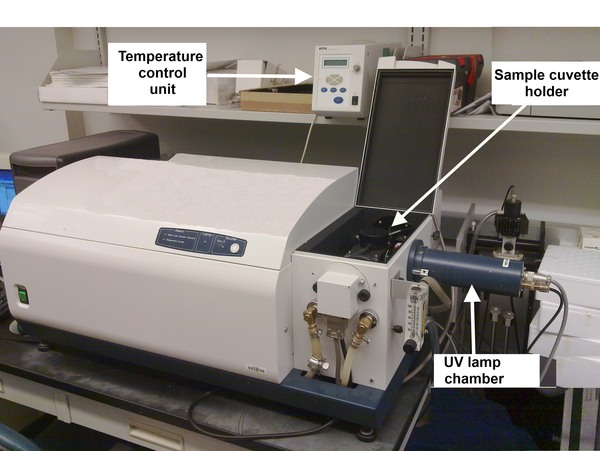
Described is a general procedure for data collection, options (time constant of instrument, wavelength interval, half-bandwidth) can be varied according to needs through the instrument controlling program.

Notification Switch
Would you like to follow the 'Physical methods in chemistry and nano science' conversation and receive update notifications?