| << Chapter < Page | Chapter >> Page > |
As mentioned earlier, most detectors used in NAA are designed to detect the gamma rays emitted from the decaying nucleus. Two widely used gamma detectors are the scintillation type and the semiconductor type. The former uses a sensitive crystal, often sodium iodide that is doped with thallium (NaI(Tl)), that emits light when gamma rays strike it. Semiconductor detectors, on the other hand, use germanium to form a diode that produces a signal in response to gamma radiation. The signal produced is proportional to the energy of the emitted gamma radiation. Both types of gamma detectors have excellent sensitivity with detection limits ranging from 0.1 to 10 6 nanogram element per gram sample, but semiconductor type detectors usually have superior resolution.
Furthermore, particles detectors designed to detect the alpha and beta particles that are emitted in nuclear decay are also available; however, gamma detectors are favorable. Particle detectors require a high vacuum since atmospheric gases in the air can absorb and affect the emission of these particles. Gamma rays are not affected in this way.
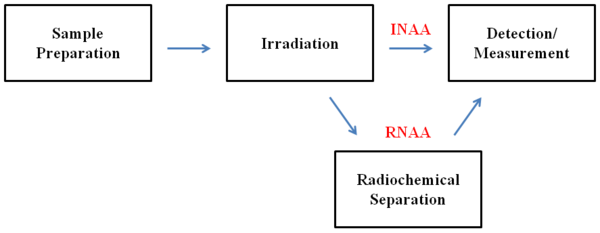
Instrumental neutron activation analysis (INAA) is the simplest and most widely used form of NAA. It involves the direct irradiation of the sample, meaning that the sample does not undergo any chemical separation or treatment prior to detection. INAA can only be used if the activity of the other radioactive isotopes in the sample does not interfere with the measurement of the element(s) of interest. Interference often occurs when the element(s) of interest are present in trace or ultratrace amounts. If interference does occur, the activity of the other radioactive isotopes must be removed or eliminated. Radiochemical separation is one way to do this. NAA that involves sample decomposition and elemental separation is known as radiochemical neutron activation analysis (RNAA). In RNAA, the interfering elements are separated from the element(s) of interest through an appropriate separation method. Such methods include extractions, precipitations, distillations, and ion exchanges. Inactive elements and matrices are often added to ensure appropriate conditions and typical behavior for the element(s) of interest. A schematic comparison of INAA and RNAA is shown below in [link] .
Another experimental parameter that must be considered is the kinetic energy of the neutrons used for irradiation. In epithermal neutron activation analysis (ENAA), the neutrons – known as epithermal neutrons – are partially moderated in the reactor and have kinetic energies between 0.5 eV to 0.5 MeV. These are lower-energy neutrons as compared to fast neutrons, which are used in fast neutron activation analysis (FNAA). Fast neutrons are high-energy, unmoderated neutrons with kinetic energies above 0.5 MeV.

Notification Switch
Would you like to follow the 'Physical methods in chemistry and nano science' conversation and receive update notifications?