| << Chapter < Page | Chapter >> Page > |
A UHV manipulator (or sample holder, see [link] ) allows an object that is inside a vacuum chamber and under vacuum to be mechanically positioned. It may provide rotary motion, linear motion, or a combination of both. The manipulator may include features allowing additional control and testing of a sample, such as the ability to apply heat, cooling, voltage, or a magnetic field. Sample heating can be accomplished by thermal radiation. A filament is mounted close to the sample and resistively heated to high temperature. In order to simplify complexity from the interaction between substrate and adsorbates, surface chemistry labs often carry out TPD experiments by choosing a substrate with single crystal surface instead of polycrystalline or amorphous substrates (see [link] ).
Before selected gas molecules are dosed to the chamber for adsorption, substrates (metal crystals) need to be cleaned through argon plasma sputtering, followed by annealing at high temperature for surface reconstruction. After these pretreatments, the system is again cooled down to very low temperature (liquid N 2 temp), which facilitating gas molecules adsorbed on the substrate surface. Adsorption is a process in which a molecule becomes adsorbed onto a surface of another phase. It is distinguished from absorption, which is used when describing uptake into the bulk of a solid or liquid phase.
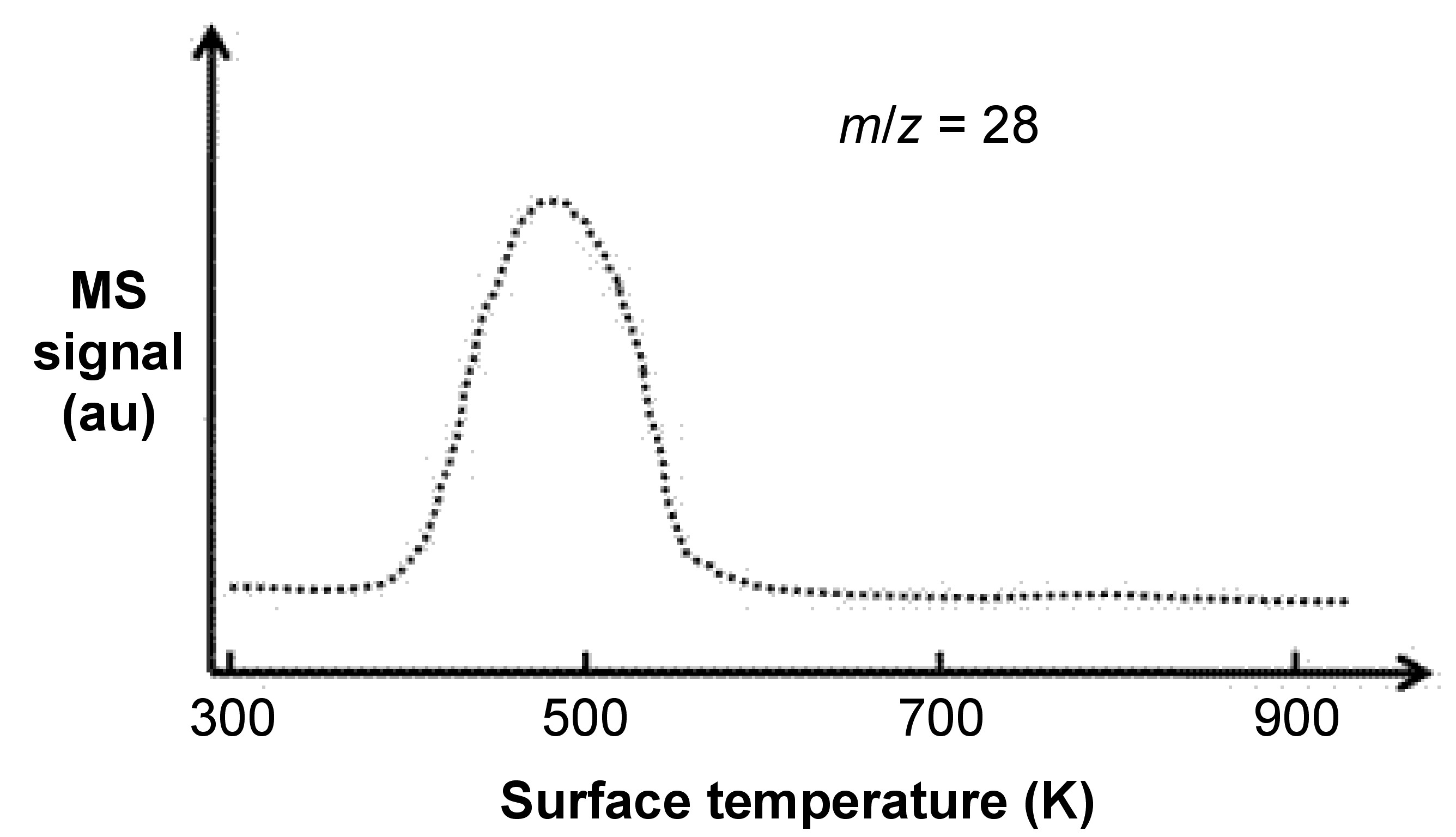
After gas molecules adsorption, now we are going to release theses adsorbates back into gas phase by programmed-heating the sample holder. A mass spectrometer is set up for collecting these desorbed gas molecules, and then correlation between desorption temperature and fragmentation of desorbed gas molecules will show us certain important information. [link] shows a typical TPD experiment carried out by adsorbing CO onto Pd(111) surface, followed by programmed-heating to desorb the CO adsorbates.
The Langmuir isotherm describes the dependence of the surface coverage of an adsorbed gas on the pressure of the gas above the surface at a fixed temperature. Langmuir isotherm is the simplest assumption, but it provides a useful insight into the pressure dependence of the extent of surface adsorption. It was Irving Langmuir who first studied the adsorption process quantitatively. In his proposed model, he supposed that molecules can adsorb only at specific sites on the surface, and that once a site is occupied by one molecule, it cannot adsorb a second molecule. The adsorption process can be represented as [link] , where A is the adsorbing molecule, S is the surface site, and A─S stands for an A molecule bound to the surface site.
In a similar way, it reverse desorption process can be represented as [link] .

Notification Switch
Would you like to follow the 'Physical methods in chemistry and nano science' conversation and receive update notifications?