| << Chapter < Page | Chapter >> Page > |
This tells us if different heating rates β are used and the left-hand side of the above equation is plotted as a function of 1/T M , we can see that a straight line should be obtained whose slope is ΔE a /R and intercept is ln(ΔE a /RA). So we are able to obtain the activation energy to desorption ΔE a and Arrhenius pre-exponential factor A.
Now let consider a second-order desorption process [link] , with a rate constant k d . We can deduce the desorption kinetics as [link] . The result is different from the first-order reaction whose T m value does not depend upon the initial coverage, the temperature of the peak T m will decrease with increasing initial surface coverage.
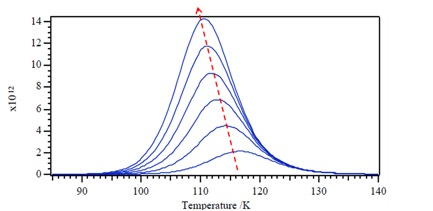
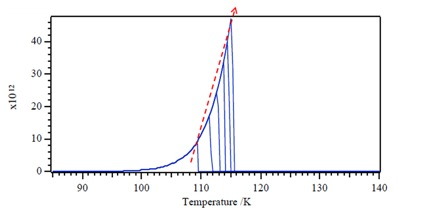
The zero-order desorption kinetics relationship as [link] . Looking at desorption rate for the zero-order reaction ( [link] ), we can observe that the desorption rate does not depend on coverage and also implies that desorption rate increases exponentially with T. Also according to the plot of desorption rate versus T, we figure out the desorption rate rapid drop when all molecules have desorbed. Plus temperature of peak, T m , moves to higher T with increasing coverage θ.
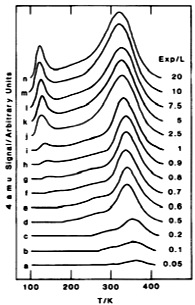
A typical TPD spectra of D 2 from Rh(100) for different exposures in Langmuirs (L = 10 -6 Torr-sec) shows in [link] . First we figure out the desorption peaks from g to n show two different desorbing regions. The higher one can undoubtedly be ascribed to chemisorbed D 2 on Rh(100) surface, which means chemisorbed molecules need higher energy used to overcome their activation energy for desorption. The lower desorption region is then due to physisorbed D 2 with much lower desorption activation energy than chemisorbed D 2 . According to the TPD theory we learnt, we notice that the peak maximum shifts to lower temperature with increasing initial coverage, which means it should belong to a second-order reaction. If we have other information about heating rate β and each T m under corresponding initial surface coverage θ then we are able to calculate the desorption activation energy E a and Arrhenius pre-exponential factor A.
Temperature-programmed desorption is an easy and straightforward technique especially useful to investigate gas-solid interaction. By changing one of parameters, such as coverage or heating rate, followed by running a serious of typical TPD experiments, it is possible to to obtain several important kinetic parameters (activation energy to desorption, reaction order, pre-exponential factor, etc). Based on the information, further mechanism of gas-solid interaction can be deduced.

Notification Switch
Would you like to follow the 'Physical methods in chemistry and nano science' conversation and receive update notifications?