| << Chapter < Page | Chapter >> Page > |
The simplest assumption is that the crystal from which the X-ray structure is determined represents the bulk sample was crystallized. With this value, either a new atom type can be generated that is the appropriate combination of the measured atom type 1 (M) and atom type 2 (M’) percent composition or two different atoms can be input with the occupancy factor set to reflect the percent composition of the bulk material. In either case the thermal parameters can be allowed to refine as usual.
The occupancy values for two atoms (M and M’) are refined (such that their sum was equal to 1), while the two atoms are constrained to have the same displacement parameters.
The occupancy values (such that their sum was equal to 1) and the displacement parameters are refined independently for the two atoms.
Once the best values for occupancy is obtained using either Methods 2 or 3, these values were fixed and the displacement parameters are allowed to refine freely.
Metal β -diketonate complexes ( [link] ) for metals in the same oxidation state are isostructural and often isomorphous. Thus, crystals obtained from co-crystallization of two or more metal β -diketonate complexes [e.g., Al(acac) 3 and Cr(acac) 3 ] may be thought of as a hybrid of the precursors; that is, the metal position in the crystal lattice may be defined as having the average metal composition.
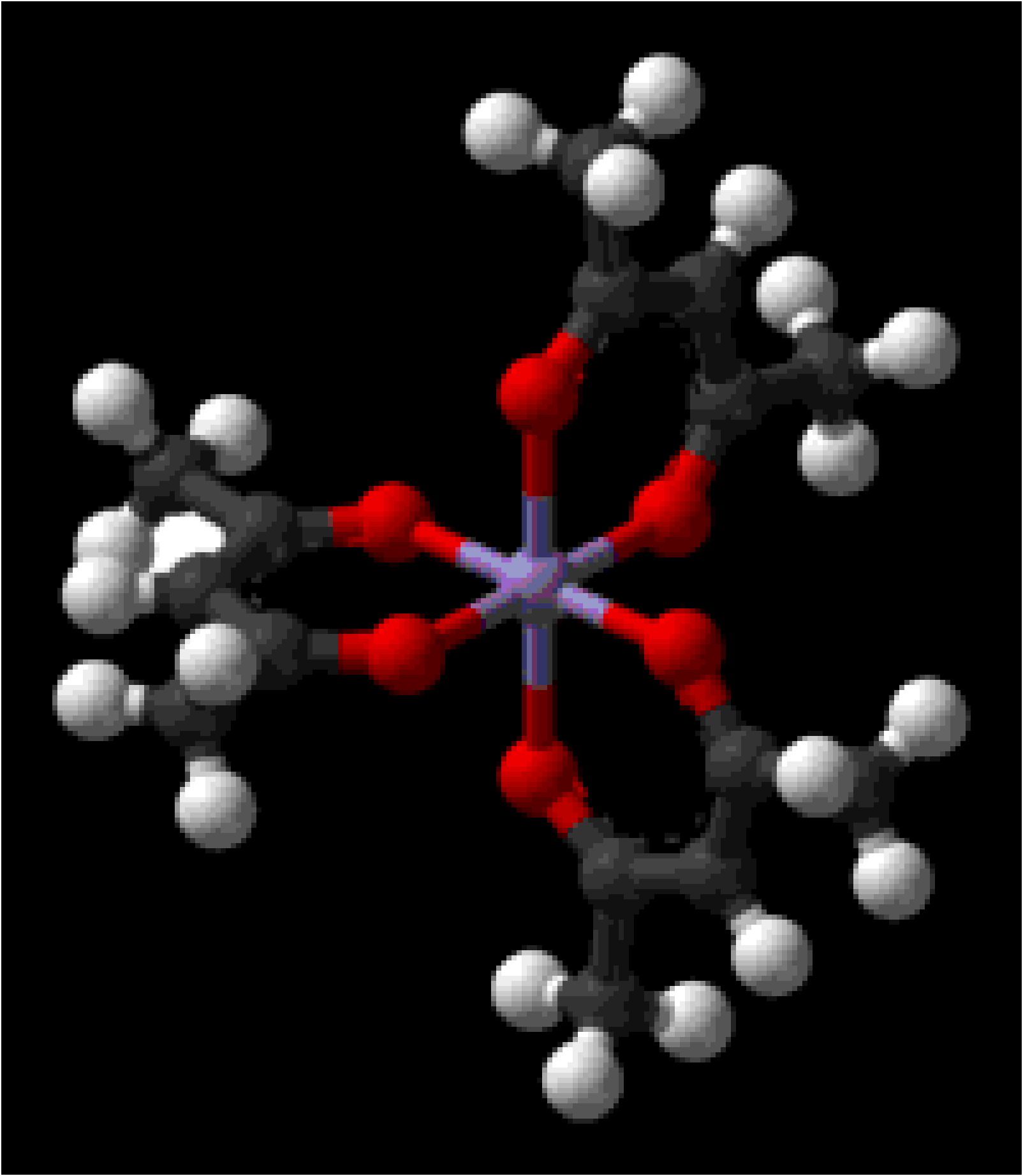
A series of solid solutions of Al(acac) 3 and Cr(acac) 3 can be prepared for study by X-ray diffraction, by the crystallization from acetone solutions of specific mixtures of Al(acac) 3 and Cr(acac) 3 ( [link] , Column 1). The pure derivatives and the solid solution, Al 1-x Cr x (acac) 3 , crystallize in the monoclinic space group P2 1 /c with Z = 4.
| Solution composition (% Cr) | WDS composition of single crystal (% Cr) | Composition as refined from X-ray diffraction (% Cr) |
| 13 | 1.9 ± 0.2 | 0 a |
| 2 | 2.1 ± 0.3 | 0 a |
| 20 | 17.8 ± 1.6 | 17.3 ± 1.8 |
| 26 | 26.7 ± 1.7 | 28.3 ± 1.9 |
| 18 | 48.5 ± 4.9 | 46.7 ± 2.1 |
| 60 | 75.1 ± 4.1 | 72.9 ± 2.4 |
| 80 | 91.3 ± 1.2 | 82.3 ± 3.1 |
Substitution of Cr for Al in the M(acac) 3 structure could possibly occur in a random manner, i.e., a metal site has an equal probability of containing an aluminum or a chromium atom. Alternatively, if the chromium had preference for specific sites a super lattice structure of lower symmetry would be present. Such an ordering is not observed since all the samples show no additional reflections other than those that may be indexed to the monoclinic cell. Therefore, it may be concluded that the Al(acac) 3 and Cr(acac) 3 do indeed form solid solutions: Al 1-x Cr x (acac) 3 .
Electron microprobe analysis, using wavelength-dispersive spectrometry (WDS), on the individual crystal from which X-ray crystallographic data was collected provides the “actual” composition of each crystal. Analysis was performed on at least 6 sites on each crystal using a 10 μm sized analysis spot providing a measure of the homogeneity within the individual crystal for which X-ray crystallographic data was collected. An example of a SEM image of one of the crystals and the point analyses is given in [link] . The data in [link] and [link] demonstrate that while a batch of crystals may contain individual crystals with different compositions, each individual crystal is actually reasonably homogenous. There is, for most samples, a significant variance between the molar Al:Cr ratio in the bulk material and an individual crystal chosen for X-ray diffraction. The variation in Al:Cr ratio within each individual crystal (±10%) is much less than that between crystals.

Notification Switch
Would you like to follow the 'Physical methods in chemistry and nano science' conversation and receive update notifications?