| << Chapter < Page | Chapter >> Page > |
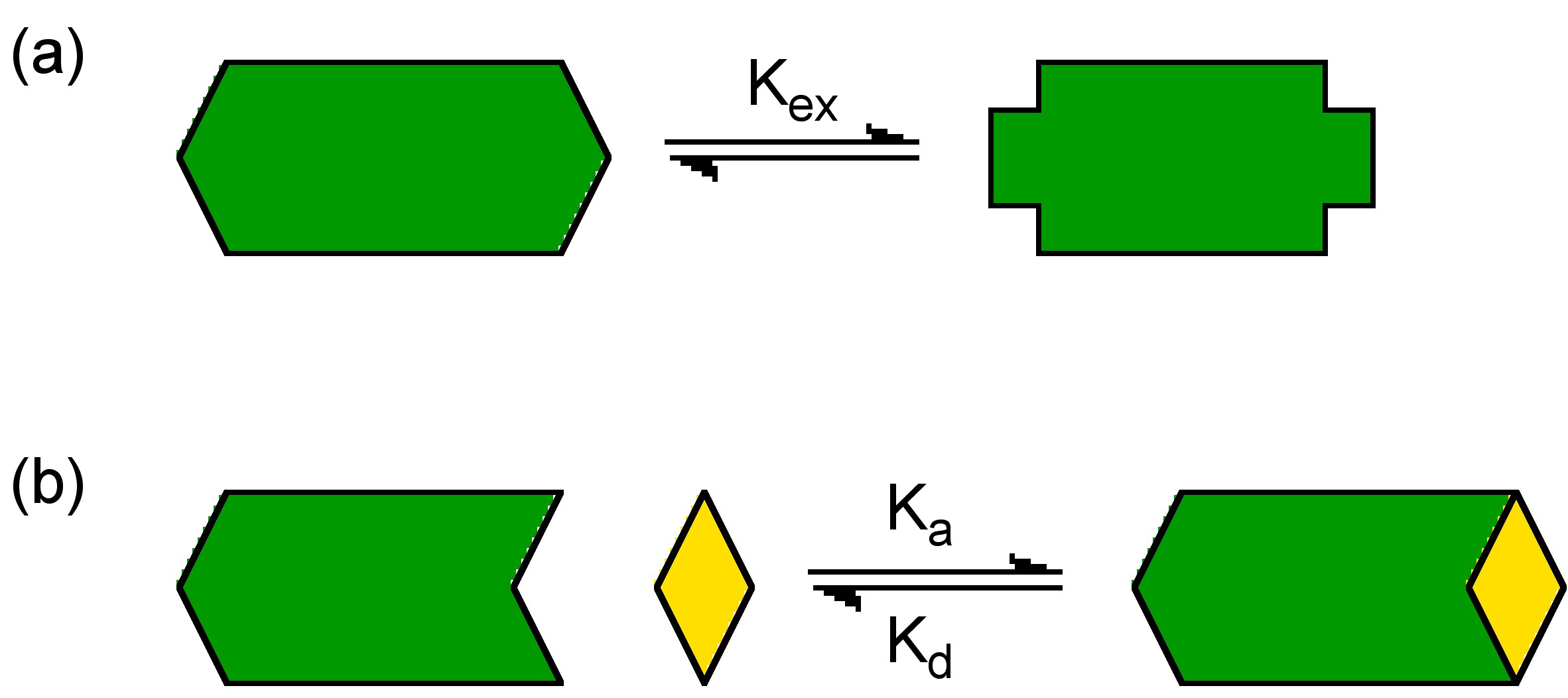
The study of conformational and chemical equilibrium is an important part of understanding chemical species in solution. NMR is one of the most useful and easiest to use tools for such kinds of work. [link] shows a pictorial representation of the process of the conformational equilibrium and chemical equilibrium. In both cases if the NMR spectra of the equilibrium species is sufficiently distinct we will have a good chance to use the NMR to investigate the system. In an equilibrium equilibrium system it is the changes in the structure/conformation of the compound that result in the variation of the peaks in the NMR spectrum. The appearance of the spectrum has a lot to do with the rate of the exchange in the equilibrium. Due to the measurement timescale of NMR when the exchange rates are slow two individual sets of peaks are observed. However, for equilibria with fast exchanges an average peak is observed.
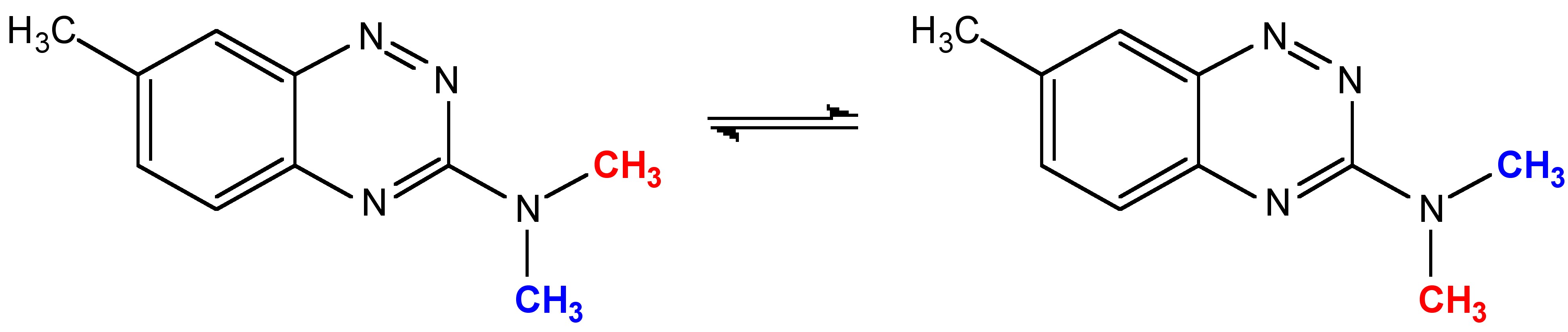
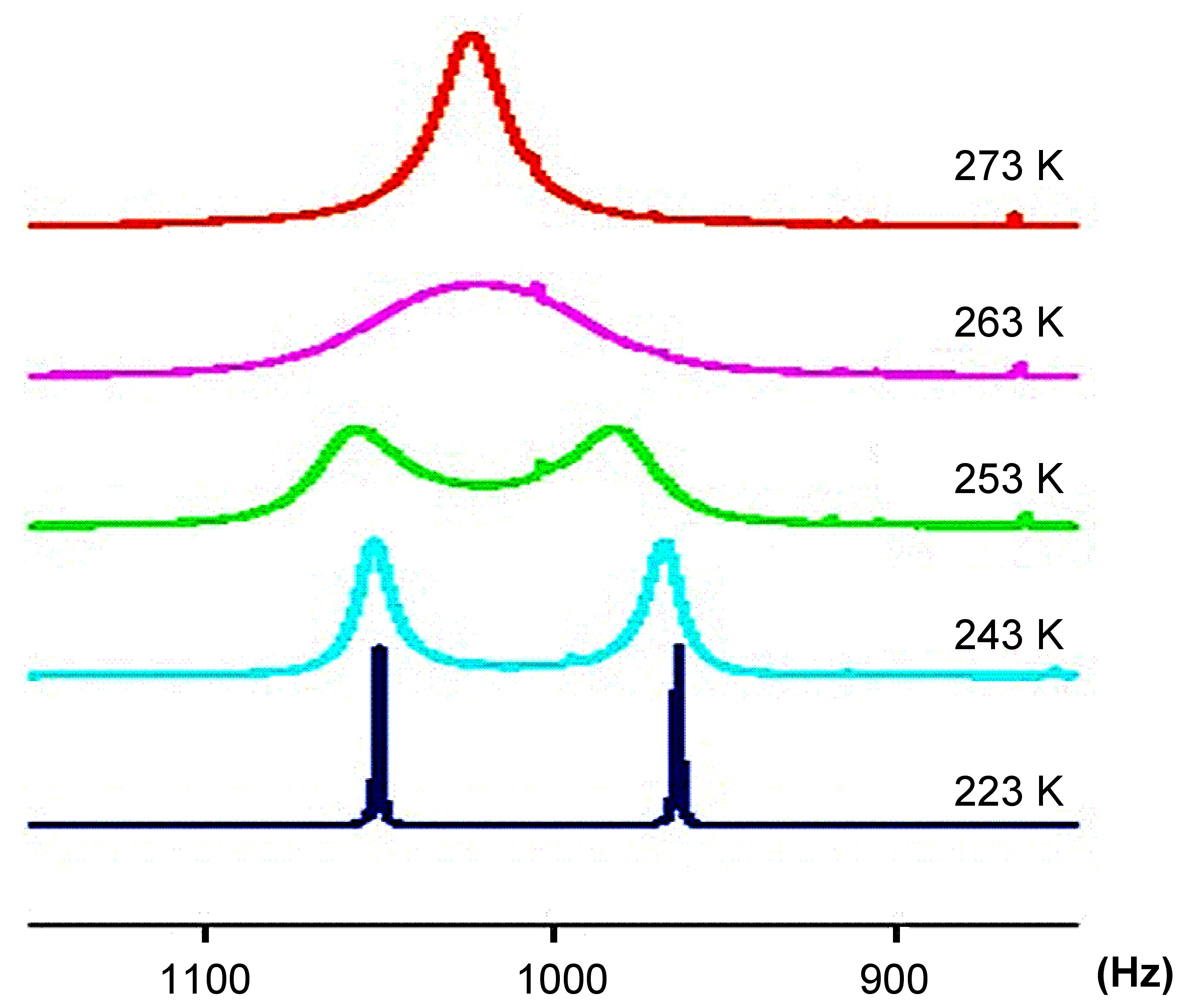
If we consider a simple system ( [link] ) as an example of how to study conformational equilibrium. In this system, the two methyl groups (one is in red, the other blue) will exchange with each other through the rotation of the C-N bond. When the speed of the rotation is fast (faster than the NMR timescale of about 10 -5 s), NMR can no longer recognize the difference of the two methyl groups, which results in an average peak in the NMR spectrum (as is shown in the red spectrum in [link] ). Conversely, when the speed of the rotation is slowed by cooling (to -50 °C) the two conformations have lifetimes significantly longer that they are observable in the NMR spectrum (as is shown by the dark blue spectrum in [link] ). The changes that occur to this spectrum with varying temperature is shown in [link] , where it is clearly seen the change of the NMR spectrum with the decreasing of temperature.
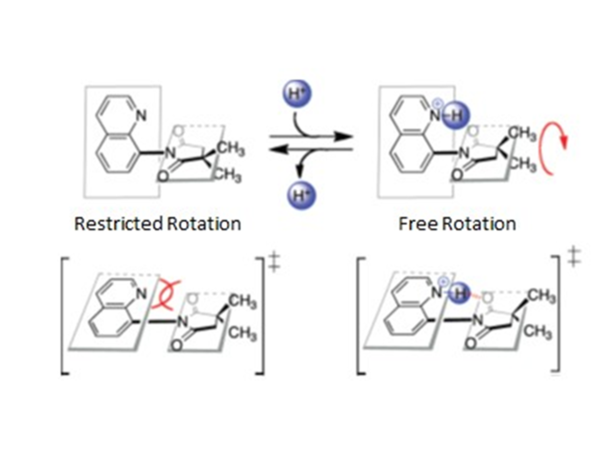
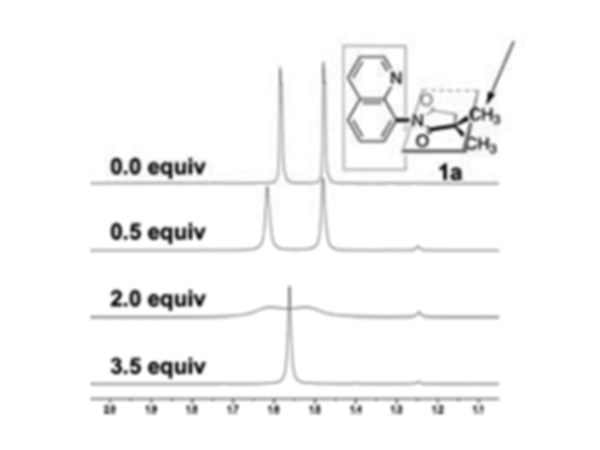
Based upon the above, it should be clear that the presence of an average or separate peaks can be used as an indicator of the speed of the rotation. As such this technique is useful in probing systems such as molecular motors. One of the most fundamental problems is to confirm that the motor is really rotating, while the other is to determine the rotation speed of the motors. In this area, the dynamic NMR measurements is an ideal technique. For example, we can take a look at the molecular motor shown in [link] . This molecular motor is composed of two rigid conjugated parts, which are not in the same plane. The rotation of the C-N bond will change the conformation of the molecule, which can be shown by the variation of the peaks of the two methyl groups in NMR spectrum. For the control of the rotation speed of this particular molecule motor, the researchers added additional functionality. When the nitrogen in the aromatic ring is not protonated the repulsion between the nitrogen and the oxygen atoms is larger which prohibits the rotation of the five member ring, which separates the peaks of the two methyl groups from each other. However, when the nitrogen is protonated, the rotation barrier greatly decreases because of the formation of a more stable coplanar transition state during the rotation process. Therefore, the speed of the rotation of the rotor dramatically increases to make the two methyl groups unrecognizable by NMR spectrometry to get an average peak. The result of the NMR spectrum versus the addition of the acid is shown in [link] , which can visually tell that the rotation speed is changing.

Notification Switch
Would you like to follow the 'Physical methods in chemistry and nano science' conversation and receive update notifications?