| << Chapter < Page | Chapter >> Page > |
Electrospray-differential mobility analysis (ES-DMA) is an analytical technique that uses first an electrospray to aerosolize particles and then DMA to characterize their electrical mobility at ambient conditions. This versatil tool can be used to quantitative characterize biomolecules and nanoparticles from 0.7 to 800 nm. In the 1980s, it was discovered that ES could be used for producing aerosols of biomacromolecules. In the case of the DMA, its predecesor was developed by Hewitt in 1957 to analize charging of small particles. The modified DMA, which is a type of ion mobility analyzer, was developed by Knutson and Whitby ( [link] ) in 1975 and later it was commercialized. Among the several designs, the cylindrical DMA has become the standard design and has been used for the obtention of monodisperse aerosols, as well as for the classification of polydisperse aerosols.

The first integration of ES with DMA ocurred in 1996 when this technique was used to determine the size of different globular proteins. DMA was refined over the past decade to be used in a wide range of applications for the characterization of polymers, viruses, bacteriophages and nanoparticle-biomolecule conjugates. Although numerous publications have reported the use of ES-DMA in medicinal and pharmaceutical applications, this present module describes the general principles of the technique and its application in the analysis of gold nanoparticles.
ES-DMA consits of an electrospray source (ES) that aerosolizes bionanoparticles and a class of ion mobility analyzer (DMA) that measures their electrical mobility by balancing electrical and drag forces on the particles. DMA continously separates particles based on their charge to size ratio. An schematic of the experimental setup for ES-DMA is shown in [link] for the analysis of gold nanoparticles.
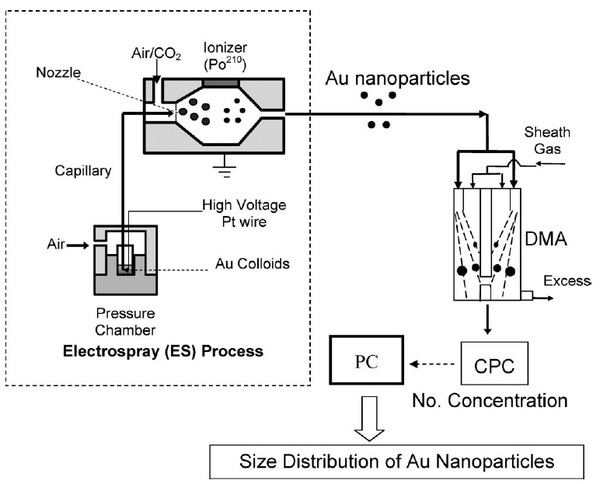
The process of analyzing particles with ES-DMA involves four steps:
First, the analyte dissolved in a volatile buffer such as ammonium acetate [NH 4 ][O 2 CCH 3 ] is placed inside a pressure chamber. Then, the solution is delivered to the nozzle through a fused silica capillary to generate multiply charged droplets. ES nebulizers produce droplets of 100-400 nm in diameter but they are highly charged.
In the next step, the droplets are mixed with air and carbon dioxide (CO 2 ) and are passed through the charge reducer or neutralizer where the solvent continues to evaporate and charge distribution decreases. The charge reducer is an ionizing α radiation source such as Po 210 that ionizes the carrier gas and reduces the net charges on the particles to a Fuchs’-Boltzmann distribution. As a result, the majority of the droplets contain single net charge particles that pass directly to the DMA. DMA separates positively or negatively charged particles by applying a negative or positive potential. [link] shows a single channel design of cylindrical DMA that is composed of two concentric electrodes between which a voltage is applied. The inner electrode is maintained at a controlled voltage from 1V to 10 kV, whereas the outer electrode is electrically grounded.

Notification Switch
Would you like to follow the 'Physical methods in chemistry and nano science' conversation and receive update notifications?